Articles
- Page Path
- HOME > J Prev Med Public Health > Volume 45(5); 2012 > Article
-
Original Article
Smoking Is Associated With Abdominal Obesity, Not Overall Obesity, in Men With Type 2 Diabetes - Ji Eun Yun1,2, Heejin Kimm1,2, Young Ju Choi3, Sun Ha Jee1,2, Kap Bum Huh3
-
Journal of Preventive Medicine and Public Health 2012;45(5):316-322.
DOI: https://doi.org/10.3961/jpmph.2012.45.5.316
Published online: September 28, 2012
1Institute for Health Promotion, Graduate School of Public Health, Yonsei University, Seoul, Korea.
2Department of Epidemiology and Health Promotion, Graduate School of Public Health, Yonsei University, Seoul, Korea.
3Huh's Diabetes Center and The 21st Century Diabetes and Vascular Research Institute, Seoul, Korea.
- Corresponding author: Sun Ha Jee, PhD. 50 Yonsei-ro, Seodaemun-gu, Seoul 120-749, Korea. Tel: +82-2-2228-1523, Fax: +82-2-365-5118, jsunha@yuhs.ac
• Received: February 15, 2012 • Accepted: August 18, 2012
Copyright © 2012 The Korean Society for Preventive Medicine
This is an Open Access article distributed under the terms of the Creative Commons Attribution Non-Commercial License (http://creativecommons.org/licenses/by-nc/3.0/) which permits unrestricted non-commercial use, distribution, and reproduction in any medium, provided the original work is properly cited.
ABSTRACT
-
Objectives
- Abdominal obesity increases mortality and morbidity from cardiovascular disease and there is a possibility that smoking effects obesity. However, previous studies concerning the effects of smoking on obesity are inconsistent. The objective of this study was to examine whether smoking is positively related to abdominal obesity in men with type 2 diabetes.
-
Methods
- Subjects consisted of 2197 type 2 diabetic patients who visited Huh's Diabetes Center from 2003 to 2009. Indices of abdominal obesity were defined as visceral fat thickness (VFT) measured by ultrasonography and waist circumference (WC). Overall obesity was defined as body mass index (BMI).
-
Results
- Statistically significant differences in WC and VFT by smoking status were identified. However, there was no statistical difference in BMI according to smoking status. Means of WC and VFT were not significantly higher in heavy smokers and lower in mild smokers. Compared to nonsmokers, the BMI confounder adjusted odds ratio and 95% confidence interval for VFT in ex-smokers and current-smokers were 1.70 (1.21 to 2.39) and 1.86 (1.27 to 2.73), respectively.
-
Conclusions
- Smoking status was positively associated with abdominal obesity in type 2 diabetic patients.
- With the obesity epidemic on the rise, research on obesity has intensified. A previous study showed, that compared to overall obesity, abdominal obesity increases the risk of metabolic diseases, such as cardiovascular disease (CVD), diabetes, hypertension, dyslipidemia, and insulin resistance [1]. In addition, it has been reported that even with a body mass index (BMI) in the normal range, a significantly higher waist circumference (WC) or waist to hip ratio (WHR) increases the possibility of complications and obesity-related diseases [2]. Despite having low BMI levels compared to Western populations, the Korean population is at risk for type 2 diabetes due to relatively high central obesity and hypertension [3]. Recently, the rate of obesity has increased in Korea. According to the Korea National Health and Nutrition Examination Survey, the overweight and obese Korean population over 20 years of age has prevalence rates of 36.7% for men and 27.4% for women [4].
- Smoking prevention and smoking cessation play important roles in improving overall health, however, many teenagers and women start smoking mistakenly believing that it will help them lose weight. In fact, it has been reported that 60% of female smokers do so to maintain or lose weight [5]. The smoking prevalence of Korean men over 30 years of age decreased from 72.37% in 1992 to 52.43% in 2006 [6], but Korea has had a relatively high level of tobacco use among males worldwide.
- The association between smoking and obesity is as important health issue because both smoking and increased body weight are independent risk factor for CVD. However, the effect on obesity according to smoking status or smoking amount is particularly controversial. According to data from the World Health Organization MONICA project (monitoring trends and determinants in cardiovascular disease), men and women who smoked generally had lower BMI than never smokers [7]. Several previous studies have been reported that among smokers a U-shaped relationship between the number of cigarettes smoked and body weight [8,9]. In contrast, some studies claim that correlations between smoking and the distribution of fat are irrelevant [10]. The association of smoking and visceral fat among type 2 diabetic patients has not been extensively researched. Such studies had relatively small sample sizes and inconsistent findings. Therefore, the objective of this study was to examine whether smoking is positively related to abdominal obesity as measured by visceral fat thickness (VFT) and WC in type 2 diabetic patients.
INTRODUCTION
- Study Subjects
- We examined diabetes patients (n=9531) from January 2003 to June 2009 at Huh's Diabetes Center, a primary health care facility in Seoul, Korea. Our analysis only included men who had a smoking habit (n=3337) because of a low smoking prevalence among women. Patient exclusion criteria included 1) diagnosis with type 1 diabetes (identified with a fasting C-peptide level <0.5 ng/mL and with either a diagnosis of diabetes before the age of 30 years or a history of diabetic ketoacidosis), 2) absence of VFT and WC measurements, 3) a history of CVD, and 4) diagnosis with any type of cancer. A total of 2197 patients with type 2 diabetes mellitus were eventually enrolled in the study. The Yonsei Medical University College of Medicine ethics committee approved the study protocol.
- Clinical and Biochemical Assessment
- Patients were diagnosed with diabetes based on the American Diabetes Association criteria. For all participating patients, the study used data collected during the patient's first visit. A single trained study staff member collected all the data, including anthropometric indices. Participants were interviewed through a structured questionnaire in order to collect lifestyle data (such as smoking habits, alcohol consumption, and exercise). They were asked to describe their smoking habit (never-smoker, ex-smoker, or current smoker), alcohol consumption (nondrinker or drinker, regardless of the amount of alcohol), exercise routine (exercising or not exercising), as well as other demographic characteristics such as age, gender, and diabetes duration, and medication. The amount and duration of smoking among current smokers was also recorded. Smoking amount was categorized as 1 to 10, 11 to 20, or ≥21. Weight and height were measured for all subjects while they were wearing light clothing and not wearing shoes. Multiple measures of obesity indices were recorded and calculated, including BMI, WC, and VFT. BMI was calculated by dividing weight in kilograms by the square of height in meters. WC was measured midway between the lower rib margin and iliac crest. VFT was measured by high-resolution ultrasonography [11-13] and defined as the distance between the anterior wall of the aorta and the internal face of the rectoabdominal muscle perpendicular to the aorta. Transverse scanning was performed using a 3.5 MHz probe to measure VFT at 1 cm above the umbilicus. Blood pressure was measured using a standard mercury sphygmomanometer after the subjects had been seated for at least 10 minutes. Blood was collected after more than 10 hours of fasting, and fasting blood glucose, glycosylated hemoglobin, total cholesterol, high-density lipoprotein (HDL), triglycerides, and low-density lipoprotein were measured.
- Statistical Analysis
- The results are presented as mean±SD or number (%).The partial correlation coefficient was used to describe the association between VFT and other continuous variables of interest, after controlling for the effect of age. Differences in general characteristics between never-smoker, ex-smoker, and current smoker groups were compared using one-way ANOVA (for continuous variables) or the chi-square test (for categorical variables). The crude or adjusted mean VFT values were categorized according to smoking status and smoking amount. Analyses were adjusted for variables such as age, duration of diabetes, alcohol consumption, exercise, and BMI. We additionally adjusted for the use of any diabetes medication (medication; no medication) and for hemoglobin A1c (HbA1c) levels, in order to control for the effects of diabetes therapy or glycemic management.
- Moreover, we analyzed the subgroup of patients (n=453) with available smoking amount data. High VFT was defined as a VFT of ≥47.6 mm [13]. The nonsmoker group included never-smokers and ex-smokers. A multivariate logistic regression model was used to test the independent association between smoking and high VFT, adjusting for potential confounding variables such as age, diabetes duration, alcohol consumption, exercise, and BMI. All analyses were conducted using the SAS version 9.1 (SAS Inc., Cary, NC, USA). All statistical tests were two-sided, and statistical significance was accepted for p<0.05.
METHODS
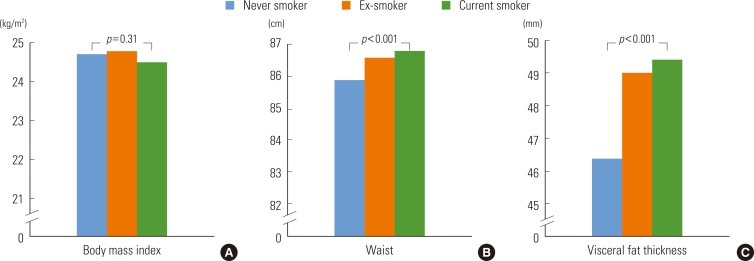
- Our results showed that among the type 2 diabetic patients participating in the study, approximately 50% were ex-smokers and 33.5% were current smokers. The mean age of participants was 55.5 years (age range, 28 to 87 years). The VFT mean values were 47.1, 49.2, and 49.8 mm for never-, ex-, and current smokers, respectively (Table 1). Current smokers had higher HbA1c and lipid profile levels, compared to never-smokers and ex-smokers. Drugs used in the treatment of type 2 diabetes include sulfonylurea, biguanide, acarbose, glitazone (TZDs), insulin, and statin. The percentage of type 2 diabetic patients who were on sulfonylurea, biguanide, acarbose, TZDs, insulin, and other hypoglycemic drugs were 51.7%, 36.7%, 12.2%, 9.7%, 10.7%, and 0.4%, respectively. The prevalence of any diabetes medication was different among the 3 groups (data not shown).
- VFT, which was determined using ultrasonography, was best correlated with WC (r=0.667, p<0.000), followed by BMI (r=0.627, p<0.000) (Table 2). The correlation between VFT and clinical laboratory values of HDL, cholesterol, triglycerides, blood pressure, and insulin was also significant.
- The mean VFT values are shown according to smoking status and amount in Table 3. Statistically significant differences in WC values were found between current smokers, ex-smokers, and never-smokers after adjusting for age, duration of diabetes, alcohol consumption, regular exercise, and BMI. Among current smokers, the number of cigarettes smoked per day also positively correlated with the abdominal obesity indicators WC and VFT, although the correlation was not statistically significant.
- The association between smoking status and abdominal obesity, after adjusting for diabetes medication and HbA1c levels, was slightly attenuated but remained significant (Figure 1). Current smokers had the highest WC and VFT values, followed by ex-smokers, and then never-smokers. The BMI values, however, were not significantly different between current, never-, and ex-smokers.
- This study examined the independent risk of high VFT levels in relation to smoking status, by using multivariate logistic regression analysis (Figure 2). The results showed that smoking significantly increased the risk of abdominal obesity, after adjusting for possible confounders such as BMI. We observed a linear increase in the odds ratio (OR) of abdominal obesity with increasing levels of both smoking status and amount. Patients who currently smoked more than 20 cigarettes per day had an adjusted OR of 1.93 (95% confidence interval, 1.16 to 3.21) compared to never-smokers.
RESULTS
- Our data indicates that type 2 diabetes patients who smoke have significantly higher central adiposity, according to VFT and WC values, than non-smokers. Heavy smokers particularly have higher central adiposity than light smokers. When further adjustment was made for diabetes medication and HbA1c levels, this association between smoking status and abdominal obesity, although slightly attenuated, remained significant. Heavy smokers had higher abdominal obesity OR values than light smokers, even after adjusting for BMI. Among current smokers, heavy smokers showed significantly higher VFT values. For instance, participants who smoked more than 20 cigarettes per day showed higher VFT values than those who smoked less than 10 cigarettes per day. However, no dose-response relationship between the number of cigarettes smoked and abdominal obesity was observed.
- Our study's consistent results demonstrate that current smokers have higher abdominal obesity than never- and ex-smokers. Shimokata et al. [14] previously reported positive association between smoking and abdominal obesity, regardless of whether BMI values were low. The WHR actually increased in patients who started smoking, despite their weight loss. However, according to data from the Scottish Health Survey, a decrease was observed in weight and BMI values of smokers compared to non-smokers [15]. Additionally, a recent study conducted in Turkey states that smoking inhibits visceral fat accumulation in women [16]. The study found that current smokers showed 26% less fat mass and 30% less visceral adipose tissue than non-smokers, although the differences were statistically insignificant for the men's data. Simon et al. [17] reported higher WHR for current smokers than subjects who quit smoking. Other studies claim that there is no significant association between smoking and fat distribution. Seidell et al. [10] state that smoking does not cause BMI increase, but that heavy smoking leads to higher WC. Moreover, one study claims that cigarette smoking is not related to a specific distribution pattern of body fat. Thus, studies report inconsistent conclusions regarding the relationship between obesity and smoking.
- Some studies that claim an association between smoking and abdominal obesity have shown higher abdominal obesity levels in smokers compared to non-smokers; however, the mechanisms responsible for those results have not been proven conclusively. Previous studies have also shown an association between smoking and hormonal changes. Smokers of 2.4 mg Federal Trade Commission nicotine cigarettes have increased plasma beta-endorphin and cortisol levels, compared to non-smokers [18]. Significantly, increased cortisol levels lead to increased insulin resistance, which is associated with abdominal fat and diabetes [19]. Many epidemiological studies have demonstrated increased levels of testosterone, free testosterone, and androgen in men smokers [20-22]. In one such study, smokers had higher mean plasma estradiol levels compared to non-smokers [23,24]. Exposure to androgens is linked to abdominal fat in premenopausal women [25]. However, a negative correlation links testosterone and abdominal fat distribution in men [26]. Therefore, the relationship between smoking, body fat distribution, and sex hormones remains unclear and may be further complicated by confounding factors such as alcohol consumption and stress levels.
- Nicotine consumption was previously shown to temporarily enhance metabolism during resting as well as during light physical activity. However, there has been no evidence that nicotine enhances human body energy expenditure in the long term. Moreover, some studies have demonstrated that smokers and non-smokers have similar basal metabolic rates. On the other hand, it has also been reported that smokers tend to weigh less than non-smokers and that smokers gain weight after quitting. Several studies have demonstrated that body weight seems to be highest in ex-smokers, lowest in current smokers, and intermediate in never-smokers [27-29]. Nevertheless, these results are controversial (not all results follow this pattern), and the biological mechanism involved has not been established. Additionally, most studies have investigated the association between smoking and obesity in healthy subjects. This association mechanism may be different in diabetes patients. Therefore, carefully designed follow-up studies of diabetic patients are required to consolidate the correlations documented in the present study.
- Nevertheless, this study has some limitations. First, we only used baseline measurements obtained during each individual's first visit, for characterization. We did not account for possible changes in smoking status or other lifestyle behaviors during follow-up; there is the possibility that diabetic patients' WC, VFT, and glycemic control-related living habits had already changed by the time of the follow-up. Second, due to the cross-sectional design, this study could not elucidate mechanisms or determine the direction of causality. Third, although we adjusted for several known potential confounders, we cannot completely rule out the possibility of residual confounding such as stress and secondhand smoke. Stress-dependent cortisol values, in particular, have been strongly associated with abdominal obesity [30]. Finally, the contribution of fat mass distribution may vary among different populations. Accordingly, our results may differ from those of studies using data obtained from women or other ethnic group participants. Therefore, further investigation, using a longitudinal study design, is needed to determine whether smoking causes abdominal obesity in general.
- This study also has several strengths. This study was performed using a relatively large cohort of type 2 diabetes performed in one institute. Additionally, both WC and VFT were measured using ultrasonography and were used as indices of abdominal obesity. Moreover, all data, including anthropometric indices, were obtained by a single trained study staff member. VFT is a simple noninvasive alternative to computed tomography and a reliable index for visceral fat measurement and identification of diabetic patients [13]. Furthermore, we assessed the association of abdominal obesity with not only smoking status but also smoking amount. Previous studies have lacked such detailed and accurate measurements of smoking amount. This study is important because it confirms a positive relationship between high smoking amount and abdominal obesity, where both are common risk factors in patients with type 2 diabetes.
- In conclusion, our study found that smoking status was closely related to abdominal obesity. Further research is needed to elucidate the association of smoking status and/or smoking patterns and abdominal obesity. The findings of this study should be cross-validated to different populations; hence, further large-scale prospective studies are needed in type 2 diabetic patients. Re-evaluation of our conclusions should be continued since smoking may influence abdominal obesity, leading to other unfavorable health outcomes.
DISCUSSION
ACKNOWLEDGEMENTS
- 1. Lamarche B. Abdominal obesity and its metabolic complications: implications for the risk of ischaemic heart disease. Coron Artery Dis 1998;9(8):473-481. 9847978ArticlePubMed
- 2. Folsom AR, Kaye SA, Sellers TA, Hong CP, Cerhan JR, Potter JD, et al. Body fat distribution and 5-year risk of death in older women. JAMA 1993;269(4):483-487. 8419667ArticlePubMed
- 3. McKeigue PM, Shah B, Marmot MG. Relation of central obesity and insulin resistance with high diabetes prevalence and cardiovascular risk in South Asians. Lancet 1991;337(8738):382-386. 1671422ArticlePubMed
- 4. Khang YH, Yun SC. Trends in general and abdominal obesity among Korean adults: findings from 1998, 2001, 2005, and 2007 Korea National Health and Nutrition Examination Surveys. J Korean Med Sci 2010;25(11):1582-1588. 21060746ArticlePubMedPMC
- 5. Kim JH, Kim HY, Song CH, Lee KM, Jeung SP. The effects of cigarette smoking on abdominal fatness. J Korean Acad Fam Med 2000;21(9):1172-1179. (Korean)
- 6. Park EJ, Koh HK, Kwon JW, Suh MK, Kim H, Cho SI. Secular trends in adult male smoking from 1992 to 2006 in South Korea: age-specific changes with evolving tobacco-control policies. Public Health 2009;123(10):657-664. 19892378ArticlePubMed
- 7. Molarius A, Seidell JC, Kuulasmaa K, Dobson AJ, Sans S. Smoking and relative body weight: an international perspective from the WHO MONICA Project. J Epidemiol Community Health 1997;51(3):252-260. 9229053ArticlePubMedPMC
- 8. Albanes D, Jones DY, Micozzi MS, Mattson ME. Associations between smoking and body weight in the US population: analysis of NHANES II. Am J Public Health 1987;77(4):439-444. 3493709ArticlePubMedPMC
- 9. Killen JD, Fortmann SP, Telch MJ, Newman B. Are heavy smokers different from light smokers? A comparison after 48 hours without cigarettes. JAMA 1988;260(11):1581-1585. 3411738ArticlePubMed
- 10. Seidell JC, Cigolini M, Deslypere JP, Charzewska J, Ellsinger BM, Cruz A. Body fat distribution in relation to physical activity and smoking habits in 38-year-old European men. The European Fat Distribution Study. Am J Epidemiol 1991;133(3):257-265. 2000843PubMed
- 11. Suzuki R, Watanabe S, Hirai Y, Akiyama K, Nishide T, Matsushima Y, et al. Abdominal wall fat index, estimated by ultrasonography, for assessment of the ratio of visceral fat to subcutaneous fat in the abdomen. Am J Med 1993;95(3):309-314. 8368228ArticlePubMed
- 12. Armellini F, Zamboni M, Rigo L, Bergamo-Andreis IA, Robbi R, De Marchi M, et al. Sonography detection of small intra-abdominal fat variations. Int J Obes 1991;15(12):847-852. 1794927PubMed
- 13. Kim SK, Kim HJ, Hur KY, Choi SH, Ahn CW, Lim SK, et al. Visceral fat thickness measured by ultrasonography can estimate not only visceral obesity but also risks of cardiovascular and metabolic diseases. Am J Clin Nutr 2004;79(4):593-599. 15051602ArticlePubMed
- 14. Shimokata H, Muller DC, Andres R. Studies in the distribution of body fat. III. Effects of cigarette smoking. JAMA 1989;261(8):1169-1173. 2915440ArticlePubMed
- 15. Akbartabartoori M, Lean ME, Hankey CR. Relationships between cigarette smoking, body size and body shape. Int J Obes (Lond) 2005;29(2):236-243. 15505632ArticlePubMed
- 16. Onat A, Ayhan E, Hergenc G, Can G, Barlan MM. Smoking inhibits visceral fat accumulation in Turkish women: relation of visceral fat and body fat mass to atherogenic dyslipidemia, inflammatory markers, insulin resistance, and blood pressure. Metabolism 2009;58(7):963-970. 19411085ArticlePubMed
- 17. Simon JA, Seeley DG, Lipschutz RC, Vittinghoff E, Browner WS. The relation of smoking to waist-to-hip ratio and diabetes mellitus among elderly women. Prev Med 1997;26(5 Pt 1):639-644. 9327471ArticlePubMed
- 18. Gilbert DG, Meliska CJ, Williams CL, Jensen RA. Subjective correlates of cigarette-smoking-induced elevations of peripheral beta-endorphin and cortisol. Psychopharmacology (Berl) 1992;106(2):275-281. 1347955ArticlePubMed
- 19. Bjorntorp P. Metabolic implications of body fat distribution. Diabetes Care 1991;14(12):1132-1143. 1773700ArticlePubMed
- 20. Deslypere JP, Vermeulen A. Leydig cell function in normal men: effect of age, life-style, residence, diet, and activity. J Clin Endocrinol Metab 1984;59(5):955-962. 6480814ArticlePubMed
- 21. Dai WS, Gutai JP, Kuller LH, Cauley JA. Cigarette smoking and serum sex hormones in men. Am J Epidemiol 1988;128(4):796-805. 3421245ArticlePubMed
- 22. Barrett-Connor E, Khaw KT. Cigarette smoking and increased endogenous estrogen levels in men. Am J Epidemiol 1987;126(2):187-192. 3605047ArticlePubMed
- 23. Lindholm J, Winkel P, Brodthagen U, Gyntelberg F. Coronary risk factors and plasma sex hormones. Am J Med 1982;73(5):648-651. 6890309ArticlePubMed
- 24. Klaiber EL, Broverman DM, Dalen JE. Serum estradiol levels in male cigarette smokers. Am J Med 1984;77(5):858-862. 6496540ArticlePubMed
- 25. Evans DJ, Hoffmann RG, Kalkhoff RK, Kissebah AH. Relationship of androgenic activity to body fat topography, fat cell morphology, and metabolic aberrations in premenopausal women. J Clin Endocrinol Metab 1983;57(2):304-310. 6345569ArticlePubMed
- 26. Seidell JC, Bjorntorp P, Sjostrom L, Kvist H, Sannerstedt R. Visceral fat accumulation in men is positively associated with insulin, glucose, and C-peptide levels, but negatively with testosterone levels. Metabolism 1990;39(9):897-901. 2202881ArticlePubMed
- 27. Kroke A, Haftenberger M, Hoffmann K, Boeing H. EPIC Working Group on Obesity, Physical Activity and SES. BMI and smoking status in the EPIC cohorts. IARC Sci Publ 2002;156: 253-256. 12484181PubMed
- 28. Lambert R, Riboli E. International Agency for Research on Cancer. Nutrition and lifestyle: opportunities for cancer prevention. 2002. Vol. 156: Lyon: International Agency for Research on Cancer; p. 253-256
- 29. Laaksonen M, Rahkonen O, Prattala R. Smoking status and relative weight by educational level in Finland, 1978-1995. Prev Med 1998;27(3):431-437. 9612833ArticlePubMed
- 30. Rosmond R, Dallman MF, Bjorntorp P. Stress-related cortisol secretion in men: relationships with abdominal obesity and endocrine, metabolic and hemodynamic abnormalities. J Clin Endocrinol Metab 1998;83(6):1853-1859. 9626108ArticlePubMed
REFERENCES
Figure 1The means of body mass index (A), waist (B), and visceral fat thickness (C) according to smoking status. Body mass index was adjusted for age, alcohol drinking, regular exercise, duration of diabetes, medication of diabetes, and hemoglobin A1C. Waist and visceral fat thickness were adjusted for age, body mass index, alcohol drinking, regular exercise, duration of diabetes, medication of diabetes, and hemoglobin A1c.

Figure 2Odds ratio and 95% confidence interval for high visceral fat thickness according to smoking status (A) and smoking amount per day (B). 1Adjusted for age, duration of diabetes, alcohol drinking, regular exercise and body mass index.
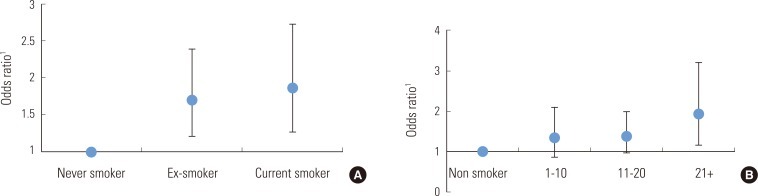

Table 1.General characteristics of study population
Table 2.Correlation between ultrasonography measured visceral fat thickness and the clinical values
|
Crude |
Age adjusted |
|||
|---|---|---|---|---|
| Correlation coefficient | p-value | Correlation coefficient1 | p-value | |
| Waist circumference | 0.668 | <0.001 | 0.668 | <0.001 |
| Body mass index | 0.628 | <0.001 | 0.631 | <0.001 |
| Systolic blood pressure | 0.181 | <0.001 | 0.191 | <0.001 |
| Diastolic blood pressure | 0.168 | <0.001 | 0.168 | <0.001 |
| Triglyceride | 0.243 | <0.001 | 0.246 | <0.001 |
| High density lipoprotein | -0.218 | <0.001 | -0.218 | <0.001 |
| Insulin | 0.275 | <0.001 | 0.275 | <0.001 |
Table 3.The mean values of visceral fat thickness according to smoking status and smoking amount
|
Waist |
Visceral fat thickness |
|||
|---|---|---|---|---|
| Crude | Adjusted | Crude | Adjusted | |
| Smoking status | ||||
| Never smoker (n = 736) | 86.4±7.7 | 85.9±0.2 | 47.1±18.4 | 46.4±0.9 |
| Ex-smoker (n = 1087) | 86.7±7.8 86.6±0.2 | 49.2±18.5 | 49.0±0.5 | |
| Current smoker (n = 736) | 86.6±8.0 86.8±0.2 | 49.8±17.7 | 49.4±0.7 | |
| p-value | 0.85 | 0.033 | 0.06 | 0.018 |
| Smoking amount (d)1 | ||||
| 1-10 (n = 121) | 85.7±0.7 | 85.9±0.4 | 48.3±1.6 | 49.2±1.3 |
| 11-20 (n = 239) | 86.3±0.5 | 86.6±0.3 | 48.7±1.1 | 48.7±0.9 |
| 21+ (n = 93) | 87.6±0.8 | 87.1±0.4 | 51.9±1.8 | 51.4±1.5 |
| p-value | 0.18 | 0.16 | 0.25 | 0.32 |
Figure & Data
References
Citations
Citations to this article as recorded by 

- Nutritional status of people who inject drugs in Coastal Kenya: a cross-sectional study
Valentine Budambula, Moses Ngari, Nancy L.M. Budambula, Aabid A. Ahmed, Tom Were
BMC Nutrition.2024;[Epub] CrossRef - Smoking and cardiovascular disease in patients with type 2 diabetes: a prospective observational study
Peder af Geijerstam, Fredrik Janryd, Fredrik H. Nyström
Journal of Cardiovascular Medicine.2023; 24(11): 802. CrossRef - Prevalence and Predictors of Combined Body Mass Index and Waist Circumference Among Indian Adults
Neha Shri, Saurabh Singh, Akancha Singh
International Journal of Public Health.2023;[Epub] CrossRef - Prevalence and related factors of abdominal obesity among urban adults aged 35 to 79 years in southwest China
Chuan Huang, Ying Zhang, Ya Liu, Jian-Xiong Liu, Yong-Mei Hu, Wei-Wei Tang, Tzung-Dau Wang, Xiao-bo Huang
Frontiers in Public Health.2023;[Epub] CrossRef - Exploring the Impact of the Obesity Paradox on Lung Cancer and Other Malignancies
Lindsay Joyce Nitsche, Sarbajit Mukherjee, Kareena Cheruvu, Cathleen Krabak, Rohit Rachala, Kalyan Ratnakaram, Priyanka Sharma, Maddy Singh, Sai Yendamuri
Cancers.2022; 14(6): 1440. CrossRef - Long-Term Adverse Effects of Cigarette Smoking on the Incidence Risk of Metabolic Syndrome With a Dose-Response Relationship: Longitudinal Findings of the Korean Genome and Epidemiology Study Over 12 Years
Ae Hee Kim, In-Ho Seo, Hye Sun Lee, Yong-Jae Lee
Endocrine Practice.2022; 28(6): 603. CrossRef - Sex-Dependent Effects of Inhaled Nicotine on the Gut Microbiome
Anna K Whitehead, Margaret C Meyers, Christopher M Taylor, Meng Luo, Scot E Dowd, Xinping Yue, Lauri O Byerley
Nicotine & Tobacco Research.2022; 24(9): 1363. CrossRef - Determinants of Metabolic Health Across Body Mass Index Categories in Central Europe: A Comparison Between Swiss and Czech Populations
Sarka Kunzova, Andrea Maugeri, Jose Medina-Inojosa, Francisco Lopez-Jimenez, Manlio Vinciguerra, Pedro Marques-Vidal
Frontiers in Public Health.2020;[Epub] CrossRef - From Pre-Diabetes to Diabetes: Diagnosis, Treatments and Translational Research
Radia Khan, Zoey Chua, Jia Tan, Yingying Yang, Zehuan Liao, Yan Zhao
Medicina.2019; 55(9): 546. CrossRef - Cigarette Smoking Is Negatively Associated with the Prevalence of Type 2 Diabetes in Middle-Aged Men with Normal Weight but Positively Associated with Stroke in Men
Su Wang, Jie Chen, Yuzhong Wang, Yu Yang, Danyu Zhang, Chao Liu, Kun Wang
Journal of Diabetes Research.2019; 2019: 1. CrossRef - Forecasting obesity prevalence in Korean adults for the years 2020 and 2030 by the analysis of contributing factors
Inkyung Baik
Nutrition Research and Practice.2018; 12(3): 251. CrossRef - Obesity in Older Type 2 Diabetic Patients: Does Working Environment Add Vulnerability?
Maria Brandão, Margarida Cardoso
International Journal of Environmental Research and Public Health.2018; 15(12): 2677. CrossRef - Smoking and the risk of type 2 diabetes
Judith Maddatu, Emily Anderson-Baucum, Carmella Evans-Molina
Translational Research.2017; 184: 101. CrossRef - Metabolic effects of smoking cessation
Kindred K. Harris, Mohan Zopey, Theodore C. Friedman
Nature Reviews Endocrinology.2016; 12(5): 299. CrossRef - Smoking status and abdominal obesity among normal- and overweight/obese adults: Population-based FINRISK study
Eeva-Liisa Tuovinen, Suoma E. Saarni, Satu Männistö, Katja Borodulin, Kristiina Patja, Taru H. Kinnunen, Jaakko Kaprio, Tellervo Korhonen
Preventive Medicine Reports.2016; 4: 324. CrossRef - Joint Association of Nicotinic Acetylcholine Receptor Variants with Abdominal Obesity in American Indians: The Strong Heart Family Study
Yun Zhu, Jingyun Yang, Fawn Yeh, Shelley A. Cole, Karin Haack, Elisa T. Lee, Barbara V. Howard, Jinying Zhao, Mohammed Akaaboune
PLoS ONE.2014; 9(7): e102220. CrossRef
 KSPM
KSPM

 PubReader
PubReader ePub Link
ePub Link Cite
Cite