Articles
- Page Path
- HOME > J Prev Med Public Health > Volume 54(6); 2021 > Article
-
Original Article
Presence of Thrombectomy-capable Stroke Centers Within Hospital Service Areas Explains Regional Variation in the Case Fatality Rate of Acute Ischemic Stroke in Korea -
Eun Hye Park1
 , Yong Jin Gil1
, Yong Jin Gil1 , Chanki Kim1
, Chanki Kim1 , Beom Joon Kim2
, Beom Joon Kim2 , Seung-sik Hwang1
, Seung-sik Hwang1
-
Journal of Preventive Medicine and Public Health 2021;54(6):385-394.
DOI: https://doi.org/10.3961/jpmph.21.329
Published online: October 22, 2021
1Department of Preventive Medicine, Graduate School of Public Health, Seoul National University, Seoul, Korea
2Department of Neurology, Seoul National University Bundang Hospital, Seongnam, Korea
- Corresponding author: Seung-sik Hwang Department of Preventive Medicine, Graduate School of Public Health, Seoul National University, 1 Gwanak-ro, Gwanak-gu, Seoul 08826, Korea E-mail: cyberdoc@snu.ac.kr
Copyright © 2021 The Korean Society for Preventive Medicine
This is an Open Access article distributed under the terms of the Creative Commons Attribution Non-Commercial License (http://creativecommons.org/licenses/by-nc/4.0/) which permits unrestricted non-commercial use, distribution, and reproduction in any medium, provided the original work is properly cited.
ABSTRACT
-
Objectives
- This study aimed to explore the status of regional variations in acute ischemic stroke (AIS) treatment and investigate the association between the presence of a thrombectomy-capable stroke center (TSC) and the case fatality rate (CFR) of AIS within hospital service areas (HSAs).
-
Methods
- This observational cross-sectional study analyzed acute stroke quality assessment program data from 262 hospitals between 2013 and 2016. TSCs were defined according to the criteria of the Joint Commission. In total, 64 HSAs were identified based on the addresses of hospitals. We analyzed the effects of structure factors, process factors, and the presence of a TSC on the CFR of AIS using multivariate logistic regression.
-
Results
- Among 262 hospitals, 31 hospitals met the definition of a TSC. Of the 64 HSAs, only 20 had a TSC. At hospitals, the presence of a stroke unit, the presence of stroke specialists, and the rate of endovascular thrombectomy (EVT) treatment were associated with reductions in the CFR. In HSAs, the rate of EVT treatment (odds ratio [OR], 0.98; 95% confidence interval [CI], 0.97 to 0.99) and the presence of a TSC (OR, 0.93; 95% CI, 0.88 to 0.99) significantly reduced the CFR of AIS.
-
Conclusions
- The presence of a TSC within an HSA, corresponding to structure and process factors related to the quality of care, contributed significantly to lowering the CFR of AIS. The CFR also declined as the rate of treatment increased. This study highlights the importance of TSCs in the development of an acute stroke care system in Korea.
- Stroke is the fourth leading cause of death in Korea, following cancer, heart disease, and pneumonia [1], and there are regional disparities in the incidence and mortality of stroke [2,3]. Strokes are classified as either ischemic or hemorrhagic stroke, depending on the mechanism of occurrence. Treatments for acute ischemic stroke (AIS) include intravenous-recombinant tissue plasminogen activator (IV-tPA) and endovascular thrombectomy (EVT) [4]. EVT was not the standard of care for acute ischemic stroke before 2015; however, EVT was included in Korea’s 2016 stroke treatment guidelines after its effectiveness at treating AIS patients with large vascular occlusions was recognized following several well-designed randomized controlled trials [5-10]. The inclusion of EVT in the guidelines does not ensure that EVT will be performed properly for patients in need given that EVT requires a readily available, 24-hour stroke team that includes vascular neurologists, skilled neurointerventionalists, and experienced technicians and nurses [11]. In short, EVT is an effective treatment, but one that requires resources. Therefore, it is not feasible for EVT treatment to be offered at all regional hospitals, and the need to establish a regional treatment delivery system according to the capability of performing EVT treatment has been raised. In the United States, stroke centers are divided into primary stroke centers (PSCs), thrombectomy-capable stroke centers (TSCs), and comprehensive stroke centers depending on the level of available stroke treatment according to the Joint Commission [12]. In Korea, regional cardio-cerebrovascular centers have been established starting in 2008 to close regional gaps and enhance the capability of regional hospitals to treat acute cardio-cerebrovascular diseases, and they have been shown to contribute to reducing mortality [13]. However, for the treatment of stroke, hospitals that provide EVT treatment still tend to be concentrated in large cities and metropolitan areas [2], and there is not yet a unified certification system in Korea to distinguish TSCs from PSCs.
- Health services research (HSR) is a field of study that examines accessibility, quality of care, and the cost of healthcare to improve health outcomes in populations [14]. Evidence for the effectiveness of new treatments is growing, but our understanding of how to efficiently deliver these treatments to patients in need is insufficient, and there is a widening gap between practice and research [15]. Among the several theoretical frameworks used in HSR, the Donabedian model describes the quality of care using a structure, process, and outcome model [16]. For example, structure factors in stroke treatment are stroke units and neurologists. Process factors include the test rate and treatment use rate, and outcome factors for determining the effectiveness of provided medical services may include the case fatality rate (CFR). Variations in treatment for AIS have been found to be caused by underuse [17]. The causes of underuse of EVT treatment have been found to be transport delays that occur during the pre-hospital stage and a lack of equipment and medical personnel for performing EVT. Due to these factors, regional disparities in AIS treatment continue to grow [2,15].
- Determining geographic units is crucial for regional variation research since the geographic units affect variation measurements [18,19]. Many variation studies have conducted their analyses of healthcare utilization using familiar administrative units. However, it is difficult to interpret and apply the results of variation measurements based on administrative units [20]. Geographic units for healthcare utilization can be defined through the relevance index, which evaluates healthcare utilization based on the patient’s residential area, and the commission index, which evaluates healthcare utilization based on the hospital’s location [21]. The Dartmouth Atlas project defined hospital service areas (HSAs) as geographic units of analysis for general hospitals and hospital referral regions for tertiary hospitals to study variations in healthcare utilization [22]. In Korea, there have been various attempts to establish HSAs, and 70 HSAs for essential care have been identified based on recent research from 2019 [23]. It is important to investigate regional variations in AIS treatment based on HSAs, which were created to reflect actual healthcare utilization in Korea. Therefore, this study aimed to examine the status of regional variations in AIS treatment and investigate the associations between the presence of a TSC within HSAs and the CFR of AIS.
INTRODUCTION
- Data Sources
- This observational study used data from the fifth, sixth, and seventh Acute Stroke Quality Assessment Program (ASQAP). The ASQAP was designed to improve the quality of care provided to acute stroke patients and has evaluated structure, process, and outcome factors of care with validated quality indicators since 2007 [24]. The investigation period was March 1 to May 31, 2013 for the fifth ASQAP, June 1 to August 31, 2014, for the sixth ASQAP, and July 1 to December 31, 2016 for the seventh ASQAP. From the fifth to seventh ASQAP, 262 hospitals that treated more than 10 patients who were hospitalized via the emergency room within 7 days of the onset of symptoms and diagnosed with acute stroke (International Statistical Classification of Diseases and Related Health Problems 10th Revision [ICD-10] code I60-I63) were included. Of the 46 794 patients treated in 262 hospitals for whom data were collected, 35 004 who were diagnosed with cerebral infarction (ICD-10 code I63) and who were aged 18 years or older were selected as the study population and aggregated into hospital units.
- HSAs were used as units of analysis of regional variation. In total, 70 HSAs were identified according to a 2019 Ministry of Health research project [23] (Supplemental Material 1). A database of HSAs was created based on hospitals’ addresses. Of the 70 HSAs, only 64 were included in the analysis. Six HSAs were excluded since the hospitals in those HSAs did not participate in the fifth, sixth, or seventh ASQAP. The map was created using an shapefile provided by the National Health Service website (KNHIS-ATLAS, http://nhiss.nhis.or.kr:8087/analysis/index.do). The map was depicted as a whole and administrative units were also shown separately on a larger scale to ensure that all regions were visible and presented accurately.
- The CFR of AIS, or the proportion of patients who died among patients diagnosed with cerebral infarction, was used as a dependent variable to measure regional variations in AIS treatment outcomes. We designated the presence of a TSC as an independent variable and defined TSCs according to the Joint Commission criteria. Applying Donabedian’s structure, process, and outcome model, a TSC was determined if the hospital or HSA contained a stroke unit with attending stroke specialists (neurology, neurosurgery, and rehabilitation medicine) and performed 15 or more EVT procedures per year. Donabedian’s structure factors and process factors were represented accordingly (Supplemental Material 2). To adjust the severity, we used the average age of the analysis unit, percentage of male patients, and the average National Institute of Health Stroke Scale (NIHSS) score as covariates, based on a prior study that developed a severity correction model using ASQAP data [25]. The NIHSS is a clinical measure in which neurological deficits are quantified from 0 point to 42 points to assess the severity of stroke at the time of hospitalization and estimate the subsequent prognosis [26]. A study comparing the prospective NIHSS and the retrospective NIHSS in Korea also confirmed that the NIHSS is both a valid and reliable indicator [27]. In particular, patients’ NIHSS scores at the time of hospitalization have been found to show a significant correlation with 30-day mortality related to AIS [28].
- Statistical Analysis
- Using exploratory data analysis, we examined regional variations in the distribution of TSCs, treatment use rate, and CFR through descriptive statistics and map images. The differences in the CFR between HSAs with and without a TSC were analyzed using mean and variance tests. The effects of TSCs on the CFR of AIS were examined at the hospital-level and HSA-level using multivariate logistic regression. The study presented the associations of both structure and process factors with the CFR as crude odds ratios (ORs) using univariate logistic regression. The adjusted OR was determined using multivariate logistic regression including the average age, percentage of male patients, and average NIHSS score. In the final model, we confirmed the effect of TSCs on the CFR of AIS patients, including the average age, percentage of male patients, average NIHSS score, and percentage of missing NIHSS scores, except for structure and process factors included in the definition of TSCs. Statistical analyses were performed using R version 3.6.1 (R Foundation for Statistical Computing, Vienna, Austria).
- Ethics Statement
- We accessed this anonymized database after submitting institutional review board approval from the Seoul National University Bundang Hospital (No. X-1902-522-902) to the Health Insurance Review & Assessment Service.
METHODS
Variables
- Among the 262 hospitals, there were 43 (16.4%) tertiary hospitals and 219 (83.6%) general hospitals. In terms of structure factors, 68 (26.0%) hospitals had stroke units and 163 (62.2%) hospitals had stroke specialists. In terms of process factors, hospitals were classified according to the annual numbers of EVT procedures performed, with 45 (17.2%) hospitals performing EVTs 15 or more times per year. In total, 31 (11.8%) TSCs met all the above conditions related to structure and process factors (Table 1). At the hospital level, the average number of AIS patients per hospital was 134, and the average age of patients was 70.2 years. The percentage of male patients was 54.7%, and the average NIHSS score was 5.67 out of 42. The percentage of missing NIHSS scores, which was one of the process factors to assess the quality of care, was 20.8%. The rate of IV-tPA treatment was 7.3%, and the rate of EVT treatment was 2.7%. The average CFR of AIS patients was 32.2% (Supplemental Material 3).
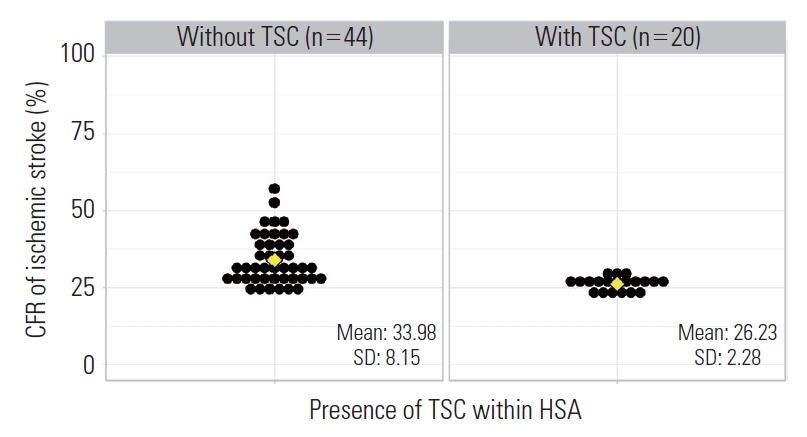
- In HSAs, the average number of hospitals that participated in the fifth, sixth, and seventh ASQAP was 4.09, and the highest quantity of hospitals was in Busan-jungbu (No. 6) with 14 hospitals (Table 1). In total, 6 HSAs—Sejong (No. 20), Icheon-gwon (No. 31), Pocheon-si (No. 32), Yeongwol-gwon (No. 35), Yeongju-gwon (No. 62), and Geochang-gwon (No. 68)—were excluded since they contained no hospitals that participated in the fifth, sixth, or seventh ASQAP (Supplemental Material 4A). The average number of TSCs within HSAs was only 0.48 (Figure 1). The average number of AIS patients per hospital was 547, and the average age was 69.67 years old. The percentage of male patients was 54.5%, and the average NIHSS score was 5.91 out of 42. The percentage of missing NIHSS scores was 20.8%. In Donghae-gwon (No. 37) and Gongju-gwon (No. 43), all of the NIHSS scores were missing (Supplemental Material 4B). The average rate of treatment with IV-tPA was 8.5% and the average rate of EVT treatment was 3.7%. Chungju-gwon (No. 40) had the highest IV-tPA treatment rate at 15.4% (Supplemental Material 4C), and Jinju-gwon (No. 65) had the highest EVT treatment rate at 12.0% (Supplemental Material 4D). The average CFR of AIS patients was 31.6% (Figure 1), the highest of which was in Naju-gwon (No. 55) at 50.6% and the lowest of which was in Seongnam-gwon (No. 22) at 21.9%.
- First, we analyzed the associations between the CFR and factors related to the quality of care in hospital settings. Structure factors such as the presence of a stroke unit and the presence of stroke specialists were considered. Process factors such as the percentage of missing NIHSS scores, the rate of IV-tPA treatment (%), the rate of EVT treatment (%), and hospital classification according to the number of annual EVT procedures were included. The results of univariate logistic regression showed a significant reduction in the CFR for all factors except for the percentage of missing NIHSS scores (OR, 1.01; 95% CI, 1.01 to 1.01) (Table 2). After severity correction with multivariate logistic regression, including the hospital-specific average age, the percentage of male patients, and the average NIHSS score, each factor showed the same direction of impact (presence of a stroke unit: OR, 0.90; 95% CI, 0.86 to 0.95; presence of stroke specialists: OR, 0.86; 95% CI, 0.79 to 0.94; rate of EVT treatment: OR, 0.98; 95% CI, 0.98 to 0.99, and the performance of 15 or more EVT procedures per year: OR, 0.82; 95% CI, 0.75 to 0.90) before the correction except for the rate of IV-tPA treatment.
- To examine the effects of TSCs on the CFR of AIS patients, the 64 HSAs were divided into 2 groups according to the presence of a TSC. In total, 20 HSAs contained a TSC and 44 HSAs did not. In terms of the distribution of TSCs within HSAs and si/do-level units (administrative units of Korea corresponding to Metropolitan cities and provinces, respectively) all of the HSAs in Seoul and Daegu contained a TSC, while none of the HSAs in Ulsan, Sejong, Chungnam, Jeonnam, Gyeongbuk, and Jeju contained a TSC (Figure 1). The average CFR was 33.98% (standard deviation [SD], 8.15) in HSAs without a TSC and 26.23% (SD, 2.28) in HSAs with a TSC. There was a significant difference in mean (mean difference, 7.75; 95% CI, 4.03 to 11.47) and variance between the groups (Figure 2). Next, we evaluated the associations between the CFR and factors related to the quality of care in HSAs. In addition, we included the presence of a TSC within HSAs as a new variable that covered structure and process factors. The results of univariate logistic regression showed a significant reduction in the CFR for all factors except for the percentage of missing NIHSS scores and the rate of IV-tPA treatment (Table 2). After severity correction with multivariate logistic regression—including the HSA-specific average age, percentage of male patients, and average NIHSS score—each factor showed the same direction of impact (presence of a stroke unit: OR, 0.93; 95% CI, 0.87 to 1.00; presence of stroke specialists: OR, 0.83; 95% CI, 0.69 to 0.99; rate of EVT treatment: OR, 0.98; 95% CI, 0.97 to 0.99; and the presence of TSCs: OR, 0.90; 95% CI, 0.86 to 0.95)—before the correction.
- Finally, we included both structure and process factors in the analysis (Table 3). In model 1, a higher average age (OR, 1.08; 95% CI, 1.06 to 1.09) and average NIHSS score (OR, 1.05; 95% CI, 1.02 to 1.07) were associated with a significantly higher CFR of AIS. Model 2 included structure factors and showed that the presence of a stroke unit (OR, 0.94; 95% CI, 0.88 to 1.00) and the presence of stroke specialists (OR, 0.83; 95% CI, 0.69 to 1.00) corresponded to a lower CFR of AIS. Model 3 included both structure and process factors and showed that the percentage of missing NIHSS scores (OR, 1.01; 95% CI, 1.00 to 1.01) corresponded to a significant increase in the CFR and that the rate of EVT treatment (OR, 0.98; 95% CI, 0.97 to 1.00) corresponded to a reduction in the CFR. The final model, Model 4, included the presence of a TSC, which covers structure and process factors and the percentage of missing NIHSS scores. Model 4 showed a significant reduction in the CFR of AIS in HSAs with a TSC compared to HSAs without a TSC (OR, 0.93; 95% CI, 0.88 to 0.99).
RESULTS
- This study examined regional variations in AIS treatment in HSA units with hospital-level data from 262 hospitals that participated in the fifth, sixth, and seventh ASQAP conducted from 2013 to 2016. The presence of TSCs in HSAs was found to have an effect on the CFR of AIS patients. The standard of care for AIS includes treatment using IV-tPA and EVT. Of the 35 004 AIS patients included in this study, 3475 (9.9%) received IV-tPA treatment and 1810 (5.2%) received EVT treatment during the survey periods of the fifth, sixth, and seventh ASQAP. To examine regional variations in AIS treatment, the level of stroke care at the hospitals within HSAs had to first be assessed. The quality of stroke care at the hospital level was divided into structure, process, and outcome factors based on Donabedian’s theoretical framework (Supplemental Material 2). Structure factors included the presence of a stroke unit and the presence of stroke specialists (neurology, neurosurgery, and rehabilitation medicine), and process factors included the percentage of missing NIHSS scores, the rate of IV-tPA treatment, and the rate of EVT treatment. The CFR of AIS was used as the outcome factor.
- The number of EVT procedures per year was used as an important criterion for defining TSCs. Many studies have concluded that a higher number of annual EVT procedures corresponds to better results, but the different number of EVT procedures performed per year was used as a threshold for considering the context of each country [29-32]. In Korea, there is not yet a unified standard to define TSCs. A recent study suggested that more than 24 EVTs per year was an indicator for identifying TSCs [33]; however, the results were derived from a study of patients who exclusively received EVT treatment, and it was therefore not appropriate to use this metric as a threshold for our study, which included patients treated with and without EVT. TSCs in this study were defined as hospitals that conducted 15 or more EVT procedures per year, per the criteria of the Joint Commission International [34]. Among the 262 hospitals included in this study, 45 (17.2%) performed 15 or more EVT procedures per year, and 31 (11.8%) had a stroke unit and stroke specialists in addition to performing 15 or more EVTs per year.
- Among the 70 identified HSAs, 6 did not contain hospitals that participated in the fifth, sixth, and seventh ASQAP, while 20 contained a TSC (Figure 1). The mean difference in the average CFR of AIS between HSAs with and without a TSC was 7.75 (95% CI, 4.03 to 11.47), and the difference of variance was also significant (Figure 2). Structure factors were analyzed using multivariate logistic regression, and the CFR was found to be lower on both the hospital and HSA levels when the process factors were well-performed. The presence of a TSC, which covers both structure and process factors, significantly lowered the CFR of AIS compared to when there was not a TSC (OR, 0.93; 95% CI, 0.88 to 0.99). Thus, this study affirms the importance of establishing TSCs in all HSAs to reduce regional variations in AIS treatment.
- This study had several limitations. First, 6 HSAs were not included in the analysis after low-level hospitals that did not meet the minimum criteria for enrollment were filtered out. Second, the analysis was conducted based on aggregated data organized by hospital units and HSA units, thus excluding the individual characteristics of patients in our analysis. Third, since the identification of HSAs was based on the addresses of hospitals rather than patients’ home addresses, the interpretation of HSA data was limited to hospitals’ characteristics according to the HSA rather than the characteristics of patients who resided in that HSA. Fourth, the period during which the survey was administered and the number of participating hospitals varied across the fifth, sixth, and seventh ASQAP, and the analysis did not consider the impact of different survey administration periods to minimize missing data and the variability of variables.
- Nevertheless, this study is meaningful due to its analysis of regional variations in AIS treatment according to HSAs rather than administrative units. This makes our results more applicable to real-world policy initiatives since HSAs are more likely to influence the implementation of policies related to regional stroke centers than administrative units. In addition, ASQAP data are more accurate and reliable for the classification of cerebrovascular diagnoses and treatments than claims data. Finally, this study covered the timely topic of regional variations in EVT treatments, which were newly included in Korea’s 2016 stroke treatment guidelines, and analyzed the impact of both structure and process factors on the CFR of AIS patients. The results of this study can thus be used as important evidence to support the establishment of regional cerebrovascular centers.
DISCUSSION
SUPPLEMENTAL MATERIALS
Supplemental Material 1.
Supplemental Material 2.
Supplemental Material 3.
Supplemental Material 4A.
Supplemental Material 4B.
Supplemental Material 4C.
Supplemental Material 4D.
-
CONFLICT OF INTEREST
The authors have no conflicts of interest associated with the material presented in this paper.
-
FUNDING
None.
Notes
ACKNOWLEDGEMENTS
-
AUTHOR CONTRIBUTIONS
Conceptualization: EHP, SSH, BJK. Data curation: EHP. Formal analysis: EHP. Funding acquisition: None. Methodology: EHP, YJG, CK, SSH, BJK. Writing – original draft: EHP. Writing – review & editing: EHP, YJG, CK, SSH, BJK.
Notes

| Characteristics | n (%) | Mean±SD | Median (Min, Max) |
|---|---|---|---|
| No. of hospitals participating ASQAP1 | 262 (100) | 4.09±2.84 | 3 (1, 14) |
| Hospital type | |||
| Tertiary hospital | 43 (16.4) | 0.67±1.21 | 0 (0, 5) |
| General hospital | 219 (83.6) | 3.42±2.06 | 3 (0, 10) |
| Structure factors2 | |||
| Stroke unit | 68 (26.0) | 1.06±1.64 | 0 (0, 8) |
| Stroke specialists3 | 163 (62.2) | 2.55±2.56 | 2 (0, 12) |
| Classification of hospitals by annual procedure of EVT | |||
| 0 | 138 (52.7) | 2.16±1.52 | 2 (0, 7) |
| 1-14 | 79 (30.1) | 1.23±1.74 | 1 (0, 10) |
| ≥15 | 45 (17.2) | 0.70±0.88 | 0.5 (0.0, 4.0) |
| TSC4 | 31 (11.8) | 0.48±0.85 | 0 (0, 4) |
| No. of acute ischemic stroke patients | 35 004 (100) | 547±590 | 407 (7, 2740) |
| Average age | - | 69.67±3.83 | 68.8 (63.9, 80.8) |
| Male | 20 161 (57.6) | 54.46±6.57 | 55.6 (33.3, 63.8) |
| Average NIHSS | - | 5.91±1.85 | 6.15 (0.00, 9.18) |
| Missing NIHSS | 2256 (6.4) | 15.79±22.01 | 4.98 (0.00, 100.00) |
| Using IV-tPA | 3475 (9.9) | 8.54±3.80 | 8.97 (0.00, 15.43) |
| Using EVT | 1810 (5.2) | 3.74±2.94 | 3.83 (0.00, 11.97) |
| Case fatality rate of acute ischemic stroke | 9451 (27.0) | 31.56±7.75 | 28.86 (21.92, 57.14) |
SD, standard deviation; Min, minimum; Max, Maximum; ASQAP, Acute Stroke Quality Assessment Program; EVT, endovascular thrombectomy; TSC, thrombectomy-capable stroke center; NIHSS, National Institute of Health Stroke Scale; IV-tPA, intravenous-recombinant tissue plasminogen activator.
1 Designed to improve the quality of care provided to acute stroke patients since 2007.
2 According to Donabedian’s theoretical framework for quality of healthcare.
3 Stroke specialists are physicians with special skills in stroke such as neurology, neurosurgery, and rehabilitation medicine.
4 TSC is defined with the presence of stroke unit, the presence of stroke specialists, and performing more than 15 EVT procedures per year according to the criteria of TSC in the Joint Commission.
| Variables |
Hospital-level (n = 262) |
Hospital service area-level (n = 64) |
||
|---|---|---|---|---|
| OR (95% CI) | aOR (95% CI)1 | OR (95% CI) | aOR (95% CI)1 | |
| Structure factors2 | ||||
| Stroke unit | 0.81 (0.77, 0.85) | 0.90 (0.86, 0.95) | 0.83 (0.79, 0.88) | 0.93 (0.87, 1.00) |
| Stroke specialists3 | 0.67 (0.62, 0.72) | 0.86 (0.79, 0.94) | 0.58 (0.49, 0.68) | 0.83 (0.69, 0.99) |
| Process factors2 | ||||
| Missing NIHSS | 1.01 (1.01, 1.01) | 1.01 (1.01, 1.01) | 1.01 (1.01, 1.01) | 1.01 (1.01, 1.01) |
| Using IV-tPA | 0.99 (0.98, 1.00) | 1.00 (0.99, 1.01) | 1.00 (0.99, 1.00) | 1.00 (0.99, 1.01) |
| Using EVT | 0.97 (0.96, 0.98) | 0.98 (0.98, 0.99) | 0.95 (0.97, 0.99) | 0.98 (0.97, 0.99) |
| Classification of hospitals by annual procedure of EVT | ||||
| 0 | 1.00 (reference) | 1.00 (reference) | - | - |
| 1-14 | 0.74 (0.69, 0.80) | 0.97 (0.88, 1.06) | - | - |
| ≥15 | 0.64 (0.56, 0.65) | 0.82 (0.75, 0.90) | - | - |
| Structure and process factors | ||||
| TSC4 | - | - | 0.82 (0.79, 0.87) | 0.90 (0.86, 0.95) |
OR, odds ratio; CI, confidence interval; aOR, adjusted odds ratio; NIHSS, National Institute of Health Stroke Scale; IV-tPA, intravenous-recombinant tissue plasminogen activator; EVT, endovascular thrombectomy; TSC, thrombectomy-capable center.
1 Adjusted for average age, percentage of men, and average NIHSS.
2 According to Donabedian’s theoretical framework for quality of healthcare.
3 Stroke specialists are physicians with special skills in stroke such as neurology, neurosurgery, and rehabilitation medicine.
4 TSC is defined with the presence of stroke unit, the presence of stroke specialists, and performing more than 15 EVT procedures per year according to the criteria of TSC in Joint Commission.
| Variables | Model 1 | Model 2 | Model 3 | Model 4 |
|---|---|---|---|---|
| Average age | 1.08 (1.06, 1.09) | 1.07 (1.05, 1.09) | 1.06 (1.04, 1.08) | 1.06 (1.04, 1.08) |
| Male | 1.01 (1.01, 1.02) | 1.01 (1.00, 1.02) | 1.01 (1.00, 1.02) | 1.01 (1.00, 1.02) |
| Average NIHSS | 1.05 (1.02, 1.07) | 1.05 (1.03, 1.08) | 1.09 (1.05, 1.13) | 1.09 (1.06, 1.13) |
| Structure factors1 | ||||
| Stroke unit | - | 0.94 (0.88, 1.00) | - | - |
| Stroke specialists2 | - | 0.83 (0.69, 1.00) | - | - |
| Process factors1 | ||||
| Missing NIHSS | - | - | 1.01 (1.00, 1.01) | 1.01 (1.00, 1.01) |
| Using IV-tPA | - | - | 1.00 (0.99, 1.01) | - |
| Using EVT | - | - | 0.98 (0.97, 1.00) | - |
| Structure and process factors | ||||
| TSC3 | - | - | - | 0.93 (0.88, 0.99) |
| AIC | 484.38 | 480.41 | 458.85 | 459.48 |
Values are presented as odds ratio (95% confidence interval).
NIHSS, National Institute of Health Stroke Scale; IV-tPA, intravenous-recombinant tissue plasminogen activator; EVT, endovascular thrombectomy; AIC, Akaike information criterion.
1 According to Donabedian’s theoretical framework for quality of healthcare.
2 Stroke specialists are physicians with special skills in stroke such as neurology, neurosurgery, and rehabilitation medicine.
3 TSC is defined with the presence of stroke unit, the presence of stroke specialists, and performing more than 15 EVT procedures per year according to the criteria of TSC in Joint Commission.
- 1. Vital Statistics Division; Statistics Korea, Kim J, Lee S, Park MS, Park S, et al. Cause-of-death statistics in 2018 in the Republic of Korea. J Korean Med Assoc 2020;63(5):286-297Article
- 2. Kim JY, Lee KJ, Kang J, Kim BJ, Kim SE, Oh H, et al. Acute stroke care in Korea in 2013-2014: national averages and disparities. J Korean Med Sci 2020;35(20):e167ArticlePubMedPMC
- 3. Lee S, Lee H, Kim H.S, Koh SB. Incidence, risk factors, and prediction of myocardial infarction and stroke in farmers: a Korean nationwide population-based study. J Prev Med Public Health 2020;53(5):313-322ArticlePubMedPMC
- 4. Kim JY, Kang K, Kang J, Koo J, Kim DH, Kim BJ, et al. Executive summary of stroke statistics in Korea 2018: a report from the Epidemiology Research Council of the Korean Stroke Society. J Stroke 2019;21(1):42-59ArticlePubMed
- 5. Goyal M, Demchuk AM, Menon BK, Eesa M, Rempel JL, Thornton J, et al. Randomized assessment of rapid endovascular treatment of ischemic stroke. N Engl J Med 2015;372(11):1019-1030ArticlePubMed
- 6. Campbell BC, Mitchell PJ, Kleinig TJ, Dewey HM, Churilov L, Yassi N, et al. Endovascular therapy for ischemic stroke with perfusion-imaging selection. N Engl J Med 2015;372(11):1009-1018ArticlePubMed
- 7. Saver JL, Goyal M, Bonafe A, Diener HC, Levy EI, Pereira VM, et al. Solitaire™ with the Intention for Thrombectomy as Primary Endovascular Treatment for Acute Ischemic Stroke (SWIFT PRIME) trial: protocol for a randomized, controlled, multicenter study comparing the Solitaire revascularization device with IV tPA with IV tPA alone in acute ischemic stroke. Int J Stroke 2015;10(3):439-448ArticlePubMedPMC
- 8. Molina CA, Chamorro A, Rovira À, de Miquel A, Serena J, Roman LS, et al. REVASCAT: a randomized trial of revascularization with SOLITAIRE FR device vs. best medical therapy in the treatment of acute stroke due to anterior circulation large vessel occlusion presenting within eight-hours of symptom onset. Int J Stroke 2015;10(4):619-626ArticlePubMed
- 9. Bracard S, Ducrocq X, Mas JL, Soudant M, Oppenheim C, Moulin T, et al. Mechanical thrombectomy after intravenous alteplase versus alteplase alone after stroke (THRACE): a randomised controlled trial. Lancet Neurol 2016;15(11):1138-1147ArticlePubMed
- 10. Hasan TF, Rabinstein AA, Middlebrooks EH, Haranhalli N, Silliman SL, Meschia JF, et al. Diagnosis and management of acute ischemic stroke. Mayo Clin Proc 2018;93(4):523-538ArticlePubMed
- 11. Papanagiotou P, Ntaios G. Endovascular thrombectomy in acute ischemic stroke. Circ Cardiovasc Interv 2018;11(1):e005362ArticlePubMed
- 12. Gorelick PB. Primary and comprehensive stroke centers: history, value and certification criteria. J Stroke 2013;15(2):78-89ArticlePubMedPMC
- 13. Cho SG, Kim Y, Choi Y, Chung W. Impact of regional cardiocerebrovascular centers on myocardial infarction patients in Korea: a fixed-effects model. J Prev Med Public Health 2019;52(1):21-29ArticlePubMed
- 14. Institute of Medicine (US) Committee on Health Services Research: Training and Work Force Issues, Thaul S, Lohr KN, Tranquada RE. Health services research: opportunities for an expanding field of inquiry. Washington, DC: National Academies Press; 1994. p. 2-3
- 15. Kaufman BG, Kucharska-Newton A, Prvu Bettger J. health services research. Stroke 2019;50(5):e121-e124ArticlePubMed
- 16. Donabedian A. Twenty years of research on the quality of medical care: 1964-1984. Eval Health Prof 1985;8(3):243-265ArticlePubMed
- 17. Johnson A, Stukel T. Medical practice variations. New York: Springer; 2016. p. 259-263
- 18. Kim AM, Park JH, Kang S, Kim Y. Evaluation of geographic indices describing health care utilization. J Prev Med Public Health 2017;50(1):29-37ArticlePubMed
- 19. Thygesen LC, Baixauli-Pérez C, Librero-López J, Martínez-Lizaga N, Ridao-López M, Bernal-Delgado E, et al. Comparing variation across European countries: building geographical areas to provide sounder estimates. Eur J Public Health 2015;25 Suppl 1: 8-14ArticlePubMed
- 20. Klauss G, Staub L, Widmer M, Busato A. Hospital service areas -- a new tool for health care planning in Switzerland. BMC Health Serv Res 2005;5: 33ArticlePubMedPMC
- 21. Griffith JR. Quantitative techniques for hospital planning and control. Lexington: Lexington Books; 1972. p. 75-78
- 22. Wennberg JE. Tracking medicine: a researcher’s quest to understand health care. New York: Oxford University Press; 2010. p. 5-8
- 23. Kim Y, Lee T, Gwak M, Shin H, Lee S, Kim J, et al. A study on the classification of health service area for essential care and the analysis of health care utilization status. Sejong: Ministry of Health and Welfare; 2019. (Korean)
- 24. Lee S, Cho M, Kim S, Ock M, Lee H, Son W, et al. A study on the improvement of acute stroke quality assessment program. 2015 [cited 2021 Jun 1]. Available from: https://repository.hira.or.kr/handle/2019.oak/1629 (Korean)
- 25. Kim K, Choi B, Park C. A study on expansion of indicators in acute stroke quality assessment program. Seoul: Health Insurance Review Agency; 2012. (Korean)
- 26. Lyden P, Lu M, Jackson C, Marler J, Kothari R, Brott T, et al. Underlying structure of the National Institutes of Health Stroke Scale: results of a factor analysis. Stroke 1999;30(11):2347-2354ArticlePubMed
- 27. Yoon SS, Bu SH, Park KC, Chang HJ, Kwon YD. Validity and reliability of retrospective NIH Stroke Scale assessment for initial stroke severity. J Korean Neurol Assoc 2006;24(1):14-20. (Korean)
- 28. Fonarow GC, Saver JL, Smith EE, Broderick JP, Kleindorfer DO, Sacco RL, et al. Relationship of national institutes of health stroke scale to 30-day mortality in medicare beneficiaries with acute ischemic stroke. J Am Heart Assoc 2012;1(1):42-50ArticlePubMedPMC
- 29. Adamczyk P, Attenello F, Wen G, He S, Russin J, Sanossian N, et al. Mechanical thrombectomy in acute stroke: utilization variances and impact of procedural volume on inpatient mortality. J Stroke Cerebrovasc Dis 2013;22(8):1263-1269ArticlePubMed
- 30. Mack WJ, Mocco J, Hirsch JA, Chen M, Elijovich L, Tarr RW, et al. Thrombectomy stroke centers: the current threat to regionalizing stroke care. J Neurointerv Surg 2018;10(2):99-101ArticlePubMed
- 31. Fargen KM, Fiorella DJ, Mocco J. Practice makes perfect: establishing reasonable minimum thrombectomy volume requirements for stroke centers. J Neurointerv Surg 2017;9(8):717-719ArticlePubMed
- 32. Gupta R, Horev A, Nguyen T, Gandhi D, Wisco D, Glenn BA, et al. Higher volume endovascular stroke centers have faster times to treatment, higher reperfusion rates and higher rates of good clinical outcomes. J Neurointerv Surg 2013;5(4):294-297ArticlePubMed
- 33. Shim DH, Kim Y, Roh J, Kang J, Park KP, Cha JK, et al. Hospital volume threshold associated with higher survival after endovascular recanalization therapy for acute ischemic stroke. J Stroke 2020;22(1):141-149ArticlePubMedPMC
- 34. Joint Commission. Thrombectomy-capable stroke center certification [cited 2020 Mar 2]. Available from: https://www.jointcommission.org/certification/certification_for_thrombectomycapable_stroke_centers.aspx
REFERENCES
Figure & Data
References
Citations

- Prioritization of Injury Prevention and Management Programs and Research and Development (R&D) Projects: Survey Using the Delphi Technique and Analytic Hierarchy Process
Won Kyung Lee, Minsu Ock, Ju Ok Park, Changsoo Kim, Beom Sok Seo, Jeehee Pyo, Hyun Jin Park, Ui Jeong Kim, Eun Jeong Choi, Shinyoung Woo, Hyesook Park
Asia Pacific Journal of Public Health.2024; 36(1): 78. CrossRef - Patterns in the number of interventionalists for endovascular treatment of acute ischemic stroke in the US
Ankita Tripathi, Laura K. Stein, Mandip S. Dhamoon
Journal of Stroke and Cerebrovascular Diseases.2023; 32(2): 106943. CrossRef - Thrombectomy-Capable Stroke Centre—A Key to Acute Stroke Care System Improvement? Retrospective Analysis of Safety and Efficacy of Endovascular Treatment in Cardiac Cathlab
Krzysztof Pawłowski, Artur Dziadkiewicz, Anna Podlasek, Jacek Klaudel, Alicja Mączkowiak, Marek Szołkiewicz
International Journal of Environmental Research and Public Health.2023; 20(3): 2232. CrossRef - COVID-19 case-fatality variations with application to the Middle East countries
Sami Khedhiri
GeoJournal.2022; 88(1): 1127. CrossRef
 KSPM
KSPM

 PubReader
PubReader ePub Link
ePub Link Cite
Cite



