Zolpidem Use and Risk of Fracture in Elderly Insomnia Patients
Article information
Abstract
Objectives
To evaluate the risk of fractures related with zolpidem in elderly insomnia patients.
Methods
Health claims data on the entire South Korean elderly population from January 2005 to June 2006 were extracted from the Health Insurance Review and Assessment Service database. We applied a case-crossover design. Cases were defined as insomnia patients who had a fracture diagnosis. We set the hazard period of 1 day length prior to the fracture date and four control periods of the same length at 5, 10, 15, and 20 weeks prior to the fracture date. Time independent confounding factors such as age, gender, lifestyle, cognitive function level, mobility, socioeconomic status, residential environment, and comorbidity could be controlled using the casecrossover design. Time dependent confounding factors, especially co-medication of patients during the study period, were adjusted by conditional logistic regression analysis. The odds ratios and their 95% confidence intervals (CIs) were estimated for the risk of fracture related to zolpidem.
Results
One thousand five hundred and eight cases of fracture were detected in insomnia patients during the study period. In our data, the use of zolpidem increased the risk of fracture significantly (adjusted odds ratio [aOR], 1.72; 95% CI, 1.37 to 2.16). However, the association between benzodiazepine hypnotics and the risk of fracture was not statistically significant (aOR, 1.00; 95% CI, 0.83 to 1.21). Likewise, the results were not statistically significant in stratified analysis with each benzodiazepine generic subgroup.
Conclusions
Zolpidem could increase the risk of fracture in elderly insomnia patients. Therefore zolpidem should be prescribed carefully and the elderly should be provided with sufficient patient education.
INTRODUCTION
The prevalence of insomnia increases with age. The trend toward emphasizing quality of life has made sound sleep an important issue. However, the changing social structure can cause sleep disturbance. In Korea, more than 20% of the population suffers from insomnia including 35% among those 65 years old and above [1,2]. As Korean society progresses from an aging society towards an aged society, the prevalence rate of insomnia and demand for insomnia control will continue to increase.
Cognitive-behavioral therapy is the first-line treatment for insomnia. However, medication is also a major treatment option offered in clinics [3,4]. Higher insomnia prevalence leads to higher hypnotic prescription and higher rates of adverse drug reactions. This could have serious results, especially in the elderly, because of their reduced drug metabolism. Even after waking up, drowsiness, dizziness, decreased alertness and concentration, and lack of coordination could endure. These residual hypnotic effects may lead to lethal results such as a fracture [5].
Nowadays, benzodiazepines and short acting non-benzodiazepine hypnotics are the main medication for controling insomnia. Even some tricyclic antidepressants and antihistamines are used to induce sleep [6-8]. Since 1960s, the benzodiazepines were established as the major drug for treating insomnia due to their marked stability compared to formerly popular hypnotics. But, after the 1980s, the development of addiction with long-term use and accidents due to residual hypnotic effects made this drug a serious social issue [9,10]. New hypnotics, such as zolpidem, zopiclone, zaleplon, and eszopiclone, the so-called "Z-drugs," appeared in the 1990s and rapidly became popular because of their much better safety record [7]. Zolpidem, a representative drug of these new hypnotics, is the most popular medicine for treating insomnia in the US at present. It is known for low tolerance, a quick induction time usually within 15 minutes, and a short half-life of two to three hours, which reduces the residual 'hangover' effects, such as sleepiness and impaired psychomotor and cognitive function, after nighttime administration that may persist into the next day [11-13]. Therefore, zolpidem was expected to be a safer alternative to the benzodiazepines, and many studies have supported its effect and safety thenceforth [14-18].
However, zolpidem could not eliminate the possibility of fracture completely [19]. Many consistent reports have claimed a fall or fracture occurred due to somnambulism (though in very low frequency) [20-23], and questions still remain about whether zolpidem is safer than the existing benzodiazepine drugs as far as risk of fracture [24]. In elderly, it was reported much serious central nervous system effects such as lack of consciousness. And risk of fracture due to fall was not much lower or even higher than the existing benzodiazepine hypnotics [25-28]. Thereupon, this study aimed to examine the risk of fracture associated with zolpidem use in elderly insomnia patients.
METHODS
Data Source
Data from the Korean Health Insurance Review and Assessment Service (HIRA) database, which contains all claims for the medication and medical procedures of approximately 50 million Koreans, between January 2005 and June 2006 were used. The database included the age, sex, prescription, and diagnosis information. The prescription data included the brand name and generic name of the drug, the date of the prescription, the amount of medication, the duration of medication, etc. Diagnoses were coded according to the International Statistical Classification of Diseases and Related Health Problems, 10th revision (ICD-10). The institutional review board of Seoul National University Hospital and Seoul National University College of Medicine approved the study protocol (protocol ID: E-1105-007-360).
Definition of Cases
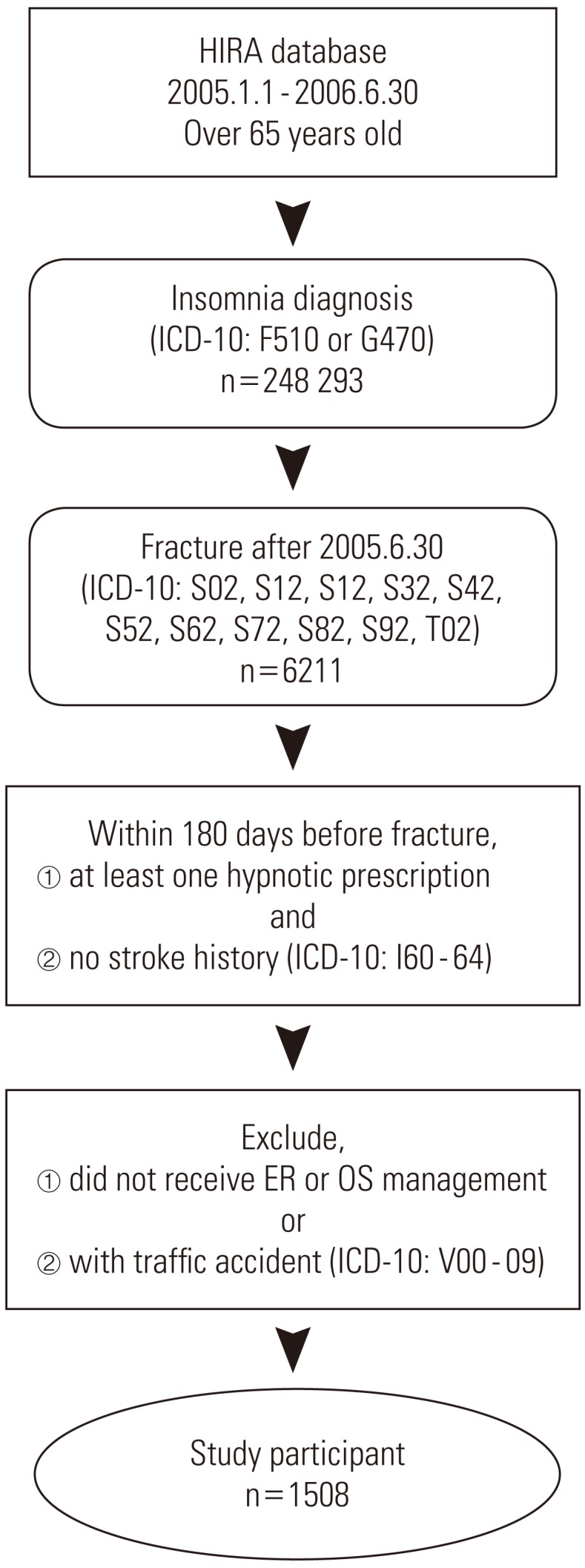
This study targeted elderly insomnia patients who visited medical clinics anywhere in South Korea for fracture as the main diagnosis from July 1, 2005 to June 30, 2006. The targeted age for the elderly was 65 years and older at the time the fracture occurred. Insomnia patients are defined as patients who were mainly or partly diagnosed with insomnia (ICD-10: F510, G470) and who were prescribed sleeping pills more than once. Among these patients, our main research targets were people who were diagnosed with fracture (ICD-10: S02, S12, S22, S32, S42, S52, S62, S72, S82, S92, T02) and visited the emergency room or were treated with orthopedics. And the first day the patient was examined was defined as the day that fracture occurred. Once fracture occurred, when a traffic accident followed (ICD-10: V00-09) or when stroke (ICD-10: 160-64) occurred during the 180 days prior to the fracture of an individual patient, then that patient was excluded from the research sample (Figure 1).
Study Design
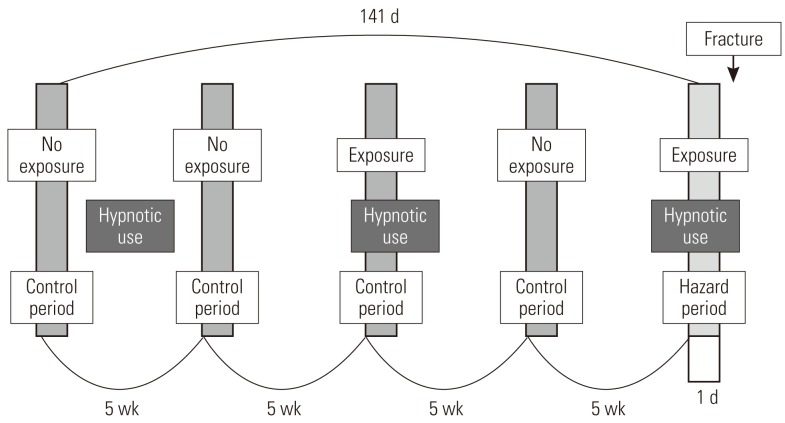
This study used a case-crossover design, and Figure 2 shows the schematization of the study. For each patient, one day right before the fracture occurred was established as the hazard period, and one day before each of 5 weeks, 10 weeks, 15 weeks, and 20 weeks from the hazard period is established as the control period, setting pairs as a ratio of 1:4 [29-31]. Fracture, by its nature, is not very likely to occur again once it occurs; thus we limited the control period to the period before the fracture occurred. It is known from existing research that a short-term harmful reaction such as a hangover usually does not occur for more than a day; thus we defined the length of the hazard period as one day before the fracture [11,19,32].
Control of Study Period
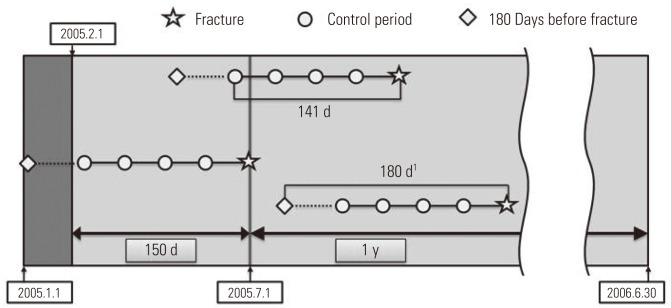
For the purpose of analysis, it was imperative that we confirmed the drug exposure information up to 141 days prior to the day the patient had a fracture, and we only targeted the cases that occurred in a specific year (from July 1, 2005 to June 30, 2006). We excluded patients from analysis targets who experienced a fracture before July 1, 2005, because we could not secure enough drug exposure information during the control period. In order to obtain information on the drugs that a patient took in a particular month, the data on fees charged in the previous month was required. So we ensured that the earliest control period of the patient did not earlier than in February of 2005. For example, if there is a patient who was prescribed a medicine called 'A' for 30 days on December 31, 2004, with only the charge data of January of 2005, it could appear as if the patient was not exposed to 'A' even though he or she was exposed until January 30, 2005. In particular, if the prescribed period was long, this problem could become worse, but in the case of the sleeping pill, which was the target of our research, every prescription period was less than 30 days, so we did not include in our research period the month that did not cover the hospital statement data up to 30 days and so forth. Therefore, in January of 2005, without establishing the control period and by collecting only the drug prescription data, we obtained the necessary data for a control period of February, 2005 (Figure 3).
Exposure Assessment
We searched every drug prescribed in Korea within the study period according to HIRA data. With the prescription data, we calculated the period of use of these drugs and the patient's exposure to zolpidem and other drugs. To adjust for irregularity of hypnotic use, we defined the period of exposure by multiplying 1.2 by the prescribed period. Considering the characteristics of sleeping pills (for example, it must be taken right before going to bed), a visit to the medical clinic due to side effects would generally take place the day after taking the drug. Thus, the very day of issuing the prescription was excluded from the exposure period. As a rule, hypnotics are prescribed for one ingredient; thus there is no reason why it should be included as a confounding variable for the analysis model of other hypnotics, but in actuality, there are cases where the clinics take doses by combining them. In these cases, other kinds of hypnotics were considered to be among the many drugs that could affect fracture, so we could adjust its effect in conditional logistic regression analysis.
Adjusting for Other Medications
Besides hypnotics, many kinds of drug are known from published studies to increase the risk of fall in elderly. These include the antipsychotics, antidepressants, antiepileptics, anxiolytics, analgesics, antiarrhythmic agents, diuretics, vasodilators, anticholinergics, anti-hypertensives and so on [33-37]. We collected the prescription data of these drugs from each patient and confirmed whether there was any exposure during the hazard period and control period. To create the final analysis model, we had to confirm which drug use was correlated with zolpidem exposure and had an association with fracture. In a chi-squared test of zolpidem exposure and other drug use, significant relevance was found in every drug (p<0.01). In conditional logistic regression analysis of each of the other drugs that may affect the risk of fracture in elder people, antidepressants, anxiolytics, diuretics, alpha blockers, beta blockers, and vasodilators did not have a significant effect on fracture in our study data. Thus we excluded them from the final analysis model.
Statistical Analysis
By adjusting for the rest of the drugs, such as antipsychotics, calcium-channel blockers, anticholinergics, antiepileptics, and analgesics, which may be confounders, we created a final analysis model that could analyze the risk ratio of fracture based on whether or not a patient was exposed to zolpidem. To compare the risk of fracture by zolpidem exposure, we generated other conditional logistic regression models that focused on benzodiazepines. Exposure to the entire group of benzodiazepine hypnotics and its generic sugroups, triazolam, lorazepam, flurazepam, flunitrazepam, midazolam, and brotizolam, were analyzed for their risk of fracture in elder insomnia patients. All analyses were done with SAS version 9.2 (SAS Inc., Cary, NC, USA).
RESULTS
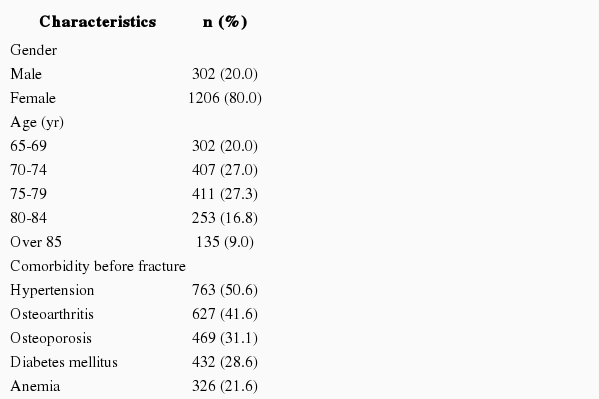
One thousand five hundred and eight people were included as subjects in the final analysis through the HIRA database. Their age, sex, and comorbidities are shown in Table 1. Only the diseases diagnosed before the fracture were accepted as comorbidities. During all hazard and control periods, 431 patients were exposed to zolpidem and 703 patients were exposed to benzodiazepine more than once. The fracture of the femur (ICD-10: S72) was the most common, and there was no association between a particular fracture site and hypnotic type.
From these 1508 subjects, after establishing one hazard period and 4 control periods, we applied conditional logistic regression analysis. The reaction risk level of fracture occurrence due to exposure to zolpidem was 1.84 and the 95% confidence interval (CI) was 1.47 to 2.30. After adjusting for the effect of other drugs that can increase the risk of fall or fracture, the reaction risk level was 1.72 and 95% CI was 1.37 to 2.16, which was also statistically significant. The reaction risk level of fracture caused by benzodiazepines (calculated using an identical method as above) was 1.12 (95% CI, 0.93 to 1.34), and after adjusting for exposure to other drugs, the reaction risk level was 1.00 (95% CI, 0.83 to 1.21), all of which were not statistically significant. The above mentioned results, even when we analyzed them by classifying drugs such as triazolam, lorazepam, flurazepam, flunitrazepam, midazolam, and brotizolam according to each ingredient, none of them had significant effects on fracture, and the same results were found even after we adjusted for exposure to other drugs. Table 2 demonstrates the results of having analyzed the risk of fracture according to the various kinds of hypnotics prescribed to elderly insomnia patients.
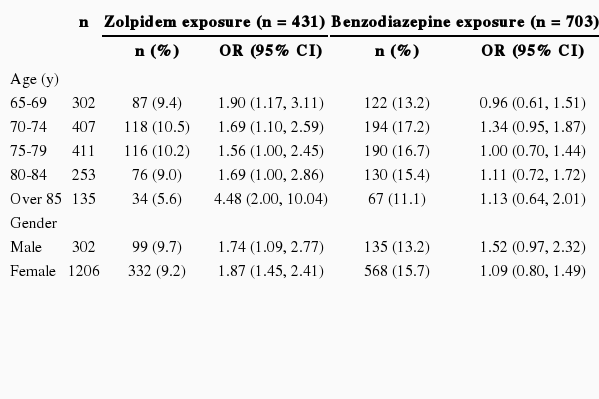
We performed sensitivity analyses to calculate the risk of fracture after subdividing study subjects according to their age and gender. Zolpidem exposure increases the risk of fracture in every age group and gender over 65 years. However, the risk increased by benzodiazepine exposure was not statistically significant in stratification analysis. Table 3 demonstrates the risk of fracture stratified by age and gender.
DISCUSSION
"Good" hypnotics are supposed to induce sleep quickly, have a great enough duration but low residual hypnotic effect, maintain sleep architecture, minimize tolerance, and dependence. Through many studies in the past, zolpidem was acknowledged to be a "better" sleeping pill compared to the benzodiazepine series that were being widely used at the time. In particular, it was expected that harmful occurrences, such as a fall due to the residual hypnotic effect of hypnotics, would occur much less among the elderly [38,39]. However, in reality, several studies have claimed that there are not so many differences in terms of fracture or fall [19,24,25,28], and the large-scale case-control study done by Wang et al. [27], which used charge data, showed that the risk of fracture due to prescribing zolpidem to elder patients is as close to twice as high, and this is even higher than benzodiazepine series sleeping pills which is 1.5 times as high. Even in our study, it was found that the risk of fracture was more than 1.7 times higher when exposed to zolpidem, and the risk of fracture when exposed to benzodiazepine series, which are known to increase the risk of fracture, was rather insignificant.
Biologically, zolpidem is known to have less risk of a residual hypnotic effect the day after exposure than benzodiazepines [11-13]. When the biological theory and epidemiological result are not matched, the research should examine with more a careful approach. For example, because benzodiazepines have a long half-life, and its hangover is much worse, leading to fewer activities the next day, thus, incidents like falls could have occurred less often. Also, there was a possibility that the risk of fracture increased for other reasons than the residual hypnotic effect of zolpidem. There have been consistent reports, although low in frequency, that claimed continuous somnambulism such as driving or sleepwalking with zolpidem [20-23]. However, we were not able to confirm that in the present study because our data have no information about the subject's activity or cause of fracture. In addition, compared to zolpidem, benzodiazepines are used for purposes other than their sleep-inducing effect such as their sedative effect, and they are taken relatively consistently [24]. In this consistent using case, because the number of patients exposed to both the hazard period and control period increases, it could have happened that the risk of fracture of benzodiazepines was not assessed appropriately [29,30].
Elderly insomnia patients usually have comorbidities and take several medications. In addition, the event of fracture could be more greatly affected by the outside environment such as living habits more than pathological progress. Therefore, we should consider many confounding variables for our analysis. The strength of this study is that we controlled for the effects of these confounding variables to the maximum degree. Within the observation period of 141 days, the possibility of varying confounding factors, including lifestyle (e.g., drinking and smoking), obesity, cognitive function level, strength of grip, mobility, socioeconomic status, and residential environment among the elderly above 65 years old, was very low. In our study, patients matched themselves as the control group; therefore, these variables were possible to control through the design of this study. Furthermore, exposure to other drugs, which continuously changed the confounding variables, could be adjusted for by using prescription data.
For the elderly, the most powerful risk factor of fracture is age [34]. In our study, the previous 5 months of each patient became the control group, and the age distribution of the patient group and control group is identical. Through this, we controlled for the effect that age has on fracture, but when we compared the risk different hypnotics have on patients, it was discovered that if the drugs (and their ingredients) prescribed were noticeably different by age then it could distort the results. In order to assess whether there were any changes according to age, we classified the subject patients into 5-year intervals, and we calculated the frequency of prescription of zolpidem and benzodiazepine series sleeping pills as well as the reaction risk level of fracture. As a result, from the patients (those people who were subjects of this research) who were 65 years old and above, we were unable to find the difference between the prescription and the reaction risk to be significant.
Out of the many adverse effects of hypnotics, fracture from falling is a serious harmful reaction, but due to its less frequent occurrence, it is not easy to prove a drug is a hazard. In this research, using large-scale administrative data, it was possible to secure a large enough number of subjects to statistically evaluate the risk of fracture due to exposure to zolpidem. However, since this study utilized only the fee data, the measurement of the result variable and exposure was done indirectly, and this study can be criticized for lacking the process to confirm the validity of these variable values.
Even if the patients were prescribed sleeping pills (according to the face-value of the prescription), we cannot confirm whether the patient actually took the pills. Even in other studies that required confirming exposure to the hypnotics using prescription data, there were many attempts to revise these differences, but those studies were unable to suggest any clear methods [9,27,40]. In the present study, we judged that the method in which the risk of exposure in proportion to the prescription period was the most rational method; thus we hypothesized that there was exposure during the period where we multiplied 1.2 by the prescription period. Using this method, we tried to minimize the differences between prescription due to irregularity of zolpidem dosage and actual exposure in this research. However, in this circumstance, even when the patient did not take the sleeping pill, the result could speak as if the patient was exposed to the risk. There is a high possibility that this circumstance could occur during the control period in many cases, and as a result, there is also a possibility that the level of hazard could have appeared lower than the actuality.
By prescription data alone, even if fracture was caused by hangover, somnambulism, or other symptoms did not caused by hypnotics, fracture used as the result variable cannot distinguish among these. In this research, in order to eliminate fracture caused by reasons other than the hypnotics as much as possible, we decided to exclude cases where they were accompanied by traffic accidents or cases where stroke occurred within 6 months since the fracture occurred in the past, but there was still a possibility that it was a fracture not caused by a harmful reaction of hypnotics, and this cannot be overlooked.
Although we assumed that the life style factor, since it is considered not to change too much as time passes, could be controlled through matching, this is not an absolute truth. For example, even if a decrease in bone density caused by a chronic drinking habit may be controlled through matching, for a person whose drinking habit is in the form of intermittent and binge drinking, occurrence of fracture due to fall are not possible to control.
Clinical trials assesses the safety of a new drug before marketing are performed by selecting research subjects who are few in numbers, relatively young, who had few comorbidities and co-medications, and who have a low risk of fall and fracture. For these reasons, it is difficult to confirm the causality of serious harmful instances that occurs rarely or confirm the safety in the elderly population, which is a vulnerable group. The present study was significant because we analyzed serious harmful reactions (although they occurred relatively rarely) without conflicts (e.g., ethical issues) by using large-scale administrative data, from an older population, which is a difficult group to study as direct research subjects.
In conclusion, it was found that zolpidem, which was expected to have few cases of side effects such as fracture, among older subjects, increases the risk of fracture by 1.7 times, and there is a lack of evidence that can claim to have a lower risk of fracture compared to existing benzodiazepine series hypnotics. Therefore, when prescribing zolpidem as sleeping pills to older insomnia patients, it is necessary to be aware of this risk, and the patient should be warned and educated.
Notes
The authors have no conflicts of interest with the material presented in this paper.