Articles
- Page Path
- HOME > J Prev Med Public Health > Volume 47(1); 2014 > Article
-
Original Article
Levothyroxine Dose and Fracture Risk According to the Osteoporosis Status in Elderly Women - Young-Jin Ko1, Ji Young Kim1, Joongyub Lee2, Hong-Ji Song3, Ju-Young Kim4, Nam-Kyong Choi2, Byung-Joo Park1,5
-
Journal of Preventive Medicine and Public Health 2014;47(1):36-46.
DOI: https://doi.org/10.3961/jpmph.2014.47.1.36
Published online: January 29, 2014
1Department of Preventive Medicine, Seoul National University College of Medicine, Seoul, Korea.
2Medical Research Collaborating Center, Seoul National University Hospital, Seoul, Korea.
3Department of Family Medicine, Hallym University College of Medicine, Chuncheon, Korea.
4Department of Family Medicine, Health Promotion Center, Seoul National University Bundang Hospital, Seongnam, Korea.
5Korea Institute of Drug Safety and Risk Management, Seoul, Korea.
- Corresponding author: Byung-Joo Park, MD, PhD. 103 Daehak-ro, Jongno-gu, Seoul 110-799, Korea. Tel: +82-2-740-8325, Fax: +82-2-747-4830, bjpark@snu.ac.kr
Copyright © 2014 The Korean Society for Preventive Medicine
This is an Open Access article distributed under the terms of the Creative Commons Attribution Non-Commercial License (http://creativecommons.org/licenses/by-nc/3.0/) which permits unrestricted non-commercial use, distribution, and reproduction in any medium, provided the original work is properly cited.
ABSTRACT
-
Objectives
- To evaluate the association between fracture risk and levothyroxine use in elderly women with hypothyroidism, according to previous osteoporosis history.
-
Methods
- We conducted a cohort study from the Korean Health Insurance Review and Assessment Service claims database from January 2005 to June 2006. The study population comprised women aged ≥65 years who had been diagnosed with hypothyroidism and prescribed levothyroxine monotherapy. We excluded patients who met any of the following criteria: previous fracture history, hyperthyroidism, thyroid cancer, or pituitary disorder; low levothyroxine adherence; or a follow-up period <90 days. We categorized the daily levothyroxine doses into 4 groups: ≤50 µg/d, 51 to 100 µg/d, 101 to 150 µg/d, and >150 µg/d. The hazard ratios (HRs) and 95% confidence intervals (CIs) were estimated with the Cox proportional hazard model, and subgroup analyses were performed according to the osteoporosis history and osteoporosis-specific drug prescription status.
-
Results
- Among 11 155 cohort participants, 35.6% had previous histories of osteoporosis. The adjusted HR of fracture for the >150 µg/d group, compared with the 51 to 100 µg/d group, was 1.56 (95% CI, 1.03 to 2.37) in osteoporosis subgroup. In the highly probable osteoporosis subgroup, restricted to patients who were concurrently prescribed osteoporosis-specific drugs, the adjusted HR of fracture for the >150 µg/d group, compared with the 51 to 100 µg/d group, was 1.93 (95% CI, 1.14 to 3.26).
-
Conclusions
- While further studies are needed, physicians should be concerned about potential levothyroxine overtreatment in elderly osteoporosis patients.
- Hypothyroidism is common in elderly women, with prevalence rates of 3% to 10% in the general population and 11% to 15% in the elderly population [1]. In Korea, a cohort study by Choi et al. [2] reported that the subclinical hypothyroidism prevalence was 18.9% in elderly Korean women. Therefore, more than 20% of elderly patients receive long-term levothyroxine replacement therapy [1]. Despite the importance of this treatment, previous studies on the association between levothyroxine use and fracture risk have reported controversial results [3-5].
- During normal aging, regulatory functions such as thyroid hormone production and degradation decrease, and therefore the required doses for elderly patients with hypothyroidism differ from those for younger patients [6,7]. Chronic under or over-replacement is common in clinical practice, and data indicate that over-replacement occurs in approximately 20% of levothyroxine patients who are treated with levothyroxine due to non-adherence or a lack of levothyroxine replacement monitoring [8-10]. Bone loss due to levothyroxine over-replacement frequently occurs in postmenopausal women [11,12]. However, evidence of an association between levothyroxine doses and fracture risk has been insufficient.
- A recent epidemiological study by Turner et al. [13] reported that high levothyroxine doses increased the fracture risk by 3-fold over that associated with low doses in elderly hypothyroidism patients. However, the authors did not consider fracture risk stratification according to the patients' osteoporosis statuses. Furthermore, the American Association of Clinical Endocrinologists (AACE) and American Thyroid Association (ATA) guidelines for hypothyroidism management did not recommend contraindicated levothyroxine doses to minimize fracture risks according to the osteoporosis status [14].
- Additionally, a study on the association between fracture risk and levothyroxine use in Korean patients has not yet been performed. Therefore, the objective of our cohort study, which employed a nationwide claims database, was to evaluate the association between levothyroxine dosage and fracture risk. Furthermore, we evaluated differences in this association according to the degree of osteoporosis.
INTRODUCTION
- Data Source and Ethical Considerations
- We accessed the Korean Health Insurance Review and Assessment Service (HIRA) database from January 2005 to June 2006. This nationwide database contains information about 4 159 309 elderly patients aged ≥65 years and 100 838 744 prescriptions. The claims database comprises inpatient and outpatient care information from all clinics and hospitals, including demographic information, diagnoses coded according to the International Classification of Disease, tenth revision (ICD-10), and prescription records such as the generic and brand names of drugs, prescription dates, durations, dosages, costs, and routes of administration [15]. This database has been used for many other epidemiological studies [16-18].
- From the HIRA database, we received data about elderly patients (aged ≥65 years) that were extracted and de-identified by HIRA to protect privacy, according to the Act on the Protection of Maintained Personal Information. The study was exempted from review by the institutional review board of the Seoul National University College of Medicine and Seoul National University Hospital because a de-identified secondary database was used.
- Cohort Construction
- The cohort participants were defined as elderly patients (aged ≥65 years) who were diagnosed with hypothyroidism (ICD-10: E01-E03) and prescribed thyroid hormone therapy from January 2005 to June 2006. Our cohort participants were restricted to elderly women. To select only patients who were prescribed levothyroxine monotherapy, we excluded patients who were prescribed liothyronine or liothyronine/levothyroxine combination therapy. We also excluded patients who met any of the following criteria: a past history of fracture (ICD-10: S22.0, S22.1, S32, S42.2-S42.4, S42.7-S42.9, S52, S62.0-S62.4, S62.8, S72, S82) prior to the first levothyroxine prescription [13]; a past history of hyperthyroidism (ICD-10: E05), thyroid cancer (ICD-10: C73), or pituitary disorder (ICD-10: E22, E23) [13]; low adherence to levothyroxine therapy; or a follow up period <90 days. Low adherence to levothyroxine therapy was defined by the proportion of days covered (PDC) and a maximum levothyroxine dose criterion of <80% and ≥300 µg/d, respectively [19,20]. PDC was calculated as the number of days covered by prescription, divided by the number of total follow-up days [21].
- Thus, we selected hypothyroidism patients who adhered well to the prescribed levothyroxine monotherapy and had no previous history of any type of fracture, hyperthyroidism, thyroid cancer, or pituitary disorder. The cohort participants were followed up from the date of first levothyroxine prescription within the study period to the date of the first fracture event, death (ICD-10: R96, R98, R99, I461), or the last date of the study period (June 30, 2006), whichever occurred first.
- Exposure and Outcome Assessment
- In Korea, a total of 18 levothyroxine formulas were introduced into the market from 2005 to 2006, and these were classified into 2 doses, 50 and 100 µg. We calculated the mean daily levothyroxine dose as the sum of each daily levothyroxine dose during the follow-up period, divided by the number of total follow-up days. If overlap occurred between prior and later prescriptions, we used the later prescription dose. As the most commonly prescribed dose was 100 µg/d and, generally, dose modifications in clinical situations were 25 µg/d, we categorized the daily levothyroxine doses into 4 groups: ≤50 µg/d, 51 to 100 µg/d, 101 to 150 µg/d, and >150 µg/d. Furthermore, the 51 to 100 µg/d group was considered a reference category when hazard ratios (HRs) were calculated.
- The first fracture diagnosed in the department of orthopedic surgery or emergency medicine after levothyroxine prescription was considered an outcome event. A fracture associated with thyroid hormone use was defined as a fracture of the wrist or forearm (ICD-10: S52, S62.0-S62.4, S62.8), shoulder or upper arm (ICD-10: S42.2-S42.4, S42.7-S42.9), thoracic spine (ICD-10: S22.0, S22.1), lumbar spine or pelvis (ICD-10: S32), hip or femur (ICD-10: S72), or lower leg or ankle (ICD-10: S82) [13]. Patients who concurrently experienced seizure (ICD-10: G40, G41, R56), trauma (ICD-10: T79, T98, V01-V99, W11-W17, W50-W52, W64, X34-X39, Y01-Y04, Y30-Y32), bone malignancy (ICD-10: C40, C41), multiple myeloma (ICD-10: C90), or pathological fracture (ICD-10: M84.4, M90.7) at any time during the study period were excluded from the outcome definition and were censored at the day on which the event occurred.
- Statistical Analyses
- We evaluated the characteristics of the cohort participants, including age, comorbidities, Charlson comorbidity scores, comedications, and the number of health service uses, with the chi-squared test and analysis of variance (ANOVA) as appropriate. If the descriptive analyses for each variable were shown to differ significantly between the groups, we performed an additional Cochran-Armitage trend test for categorical variables or Duncan's test for continuous variables.
- During the study period, we considered comorbidities and comedications as the confounding factors of the association between levothyroxine use and fracture risk. Comorbidities possibly related to the fracture risk were identified in claims by the ICD-10 codes. These comorbidities included osteoporosis (M81, M82), malignant neoplasm (C), diabetes mellitus (DM, E10-E14), hypoparathyroidism (E20), Cushing's syndrome (E24), adrenal disorder (E25-E27), chronic obstructive pulmonary disease (COPD, J40-J44), asthma (J45), chronic liver disease (B18, B19, K70-K77), chronic renal failure (N18, N19), heart failure (I50), ischemic heart disease (I20-I25), arrhythmia (I44-I49), dementia (F00-F03), stroke (I60-I64), and epilepsy (G40, G41). Comedications such as corticosteroids, anti-coagulants, anti-epileptics, anti-depressants, benzodiazepines, proton pump inhibitors (PPI), and thiazolidinediones were considered potential risk factors for fracture. In contrast, bisphosphonates and raloxifene were considered protective factors against fracture [22].
- The Cox proportional hazard model was used to calculate the HRs and 95% confidence intervals (CIs). The above-listed factors were considered possible confounders in the multivariate models because they were well-known fracture risk factors. When analyzing the fracture risk, we considered the left truncation of data, using the SAS survival data analysis function [23]. We performed subgroup analyses according to the previous histories of osteoporosis diagnoses and accordingly divided the patients into 2 subgroups: the osteoporosis subgroup and the non-osteoporosis subgroup. The osteoporosis subgroup was further divided into 2 groups according to the osteoporosis-specific drug prescription: the highly probable osteoporosis subgroup included patients who were prescribed bisphosphonate or raloxifene, while the probable osteoporosis subgroup included patients who were not prescribed bisphosphonate or raloxifene. These operational definitions of osteoporosis statuses in our study were determined from an algorithm that was formerly applied to ascertainments of osteoporosis in other HIRA database studies [18].
- We calculated the incidence rates among the total elderly women in our dataset, the eligible cohort participants, and each subgroup stratified by osteoporosis statuses and daily levothyroxine dose groups. Furthermore, these incidence rates were standardized to the age distribution of all Korean elderly women in 2005.
- All statistical analyses were performed with the SAS version 9.3 (SAS Inc., Cary, NC, USA).
METHODS
- Study Population
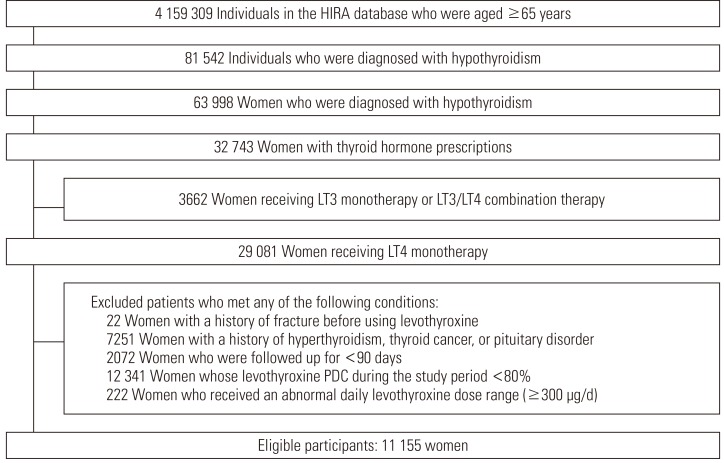
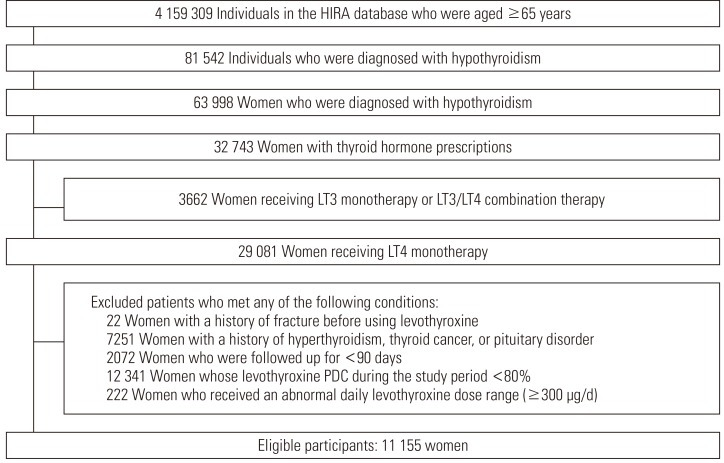
- Of the 4 159 309 elderly patients in the database, 81 542 were diagnosed with hypothyroidism during the study period, 63 998 of whom were women. Among the hypothyroidism patients, 32 743 women were prescribed thyroid hormone therapy. We selected the 29 081 women who were prescribed levothyroxine monotherapy by excluding 3662 women who were prescribed liothyronine monotherapy or liothyronine/levothyroxine combination therapy. Next, we applied the exclusion criteria, and 11 155 women were finally included as cohort participants (Figure 1). Among the cohort participants, the median and interquartile ranges of follow-up duration were 514 days and 451 to 533 days. Among those, 256 patients experienced fractures during the study period.
- Baseline Characteristics of the Participants According to Daily Levothyroxine Dose and Osteoporosis History
- Table 1 shows the differences in the baseline characteristics among the daily levothyroxine dose groups. Regarding the daily levothyroxine doses among the total cohort participants, the median was 95.7 µg/d, and the interquartile range was 81.3 to 100.0 µg/d. After categorization, 6097 patients (54.7%) were prescribed doses between 51 µg/d and 100 µg/d. Furthermore, 2296 (20.6%), 1674 (15.0%), and 1088 (9.8%) participants were prescribed daily levothyroxine doses of ≤50 µg/d, 101 to 150 µg/d, and >150 µg/d, respectively. The patients in the lowest dose group (≤50 µg/d) had a higher prevalence of cardiovascular problems, including heart failure, ischemic heart failure, arrhythmia, and stroke, than did those in the higher dose groups. In contrast, the highest dose group (>150 µg/d) had a higher prevalence of malignancy and hypoparathyroidism. However, the rates of previous histories of osteoporosis diagnosis and bisphosphonate prescriptions were not significantly different between the levothyroxine dose groups (p=0.46 and p=0.36, respectively).
- We analyzed differences in the baseline characteristics of the subgroups according to the previous histories of osteoporosis diagnosis and osteoporosis-specific drug prescriptions (Table 2). Individuals in the highly probable osteoporosis subgroup had a higher prevalence of Cushing syndrome, adrenal disorders, COPD, and asthma, higher Charlson comorbidity scores, and more prescriptions for glucocorticoids, anti-epileptics, anti-depressants, benzodiazepines, and PPI, as well as higher numbers of health service uses than those in other subgroups, and all of the p-values for the trends associated with the above-listed factors were <0.01. In contrast, the non-osteoporosis subgroup had a higher prevalence of chronic kidney diseases and heart failure, and the p-values for these trends were <0.01 and 0.02, respectively.
- Association Between the Daily Levothyroxine Dose and Fracture Risk
- Compared to the reference group (51 to 100 µg/d), the fracture risks in the ≤50 µg/d, 101 to 150 µg/d, and >150 µg/d groups were not significant in the total cohort participants. In a subgroup analysis according to osteoporosis diagnoses, the fracture risk of the highest daily dose group (>150 µg/d) was significantly higher than that of the reference group in the osteoporosis subgroup (adjusted HR, 1.56; 95% CI, 1.03 to 2.37) (Table 3). After we divided the osteoporosis subgroup into 2 subgroups (highly probable osteoporosis and probable osteoporosis subgroup), the fracture risk of the highest daily dose group was significantly increased only in the highly probable osteoporosis subgroup (adjusted HR, 1.93; 95% CI, 1.14 to 3.26). Furthermore, the fracture risk trend in the highly probable osteoporosis subgroup was correlated linearly with the daily levothyroxine dose (p for trend: 0.03).
RESULTS
- In this nationwide retrospective cohort study on the association between the levothyroxine dose and fracture risk among elderly women (aged ≥65 years), we found a significant association between a higher dose of levothyroxine (>150 µg/d) and fracture risk in the highly probable osteoporosis subgroup. This result suggests that high-dose levothyroxine treatment might increase the fracture risk in severely osteoporotic patients. However, a levothyroxine treatment ≤150 µg/d was not associated with a fracture risk, regardless of osteoporosis status.
- When we compared the age-standardized incidence rates among levothyroxine doses of >150 µg/d in the highly probable osteoporosis subgroup, eligible cohort participants, and all elderly women in our dataset, the incidence rate ratios of the >150 µg/d levothyroxine group were 2.54 when compared to the eligible cohort participants and 1.72 when compared to all elderly women in our dataset.
- The significantly increased fracture risk in severely osteoporotic patients agreed with those reported in previous studies. Evidence about the association between levothyroxine replacement and reduced bone mineral density (BMD) was well supported. Faber and Galløe [11] reported in a meta-analysis of 13 studies that women with an average age of 39.6 years who were treated with 164 µg of levothyroxine per day had a 2.67% lower BMD than that of the controls. Uzzan et al. [12] also reported in a meta-analysis of 25 studies that bone losses caused by long-term levothyroxine use in postmenopausal women significantly decreased BMDs by 7% in the lumbar spine and 9% in the femoral neck (mean age, 61.1 years; mean follow-up duration, 9.6 years). Furthermore, several researchers reported that patients who received levothyroxine replacement therapy, especially postmenopausal women, had an increased fracture risk. Flynn et al. [4] conducted a retrospective cohort study of the association between the occurrence of adverse outcomes such as cardiac and osteoporotic events and thyroid-stimulating hormone (TSH) concentrations in patients who were prescribed levothyroxine replacement therapy. The TSH-suppressed patients, represented as high-dose levothyroxine prescriptions, had a 2.02-fold increase (95% CI, 1.55 to 2.62) in the risk of osteoporotic fractures. In a nested case-control study conducted in Canada by Turner et al. [13], 213 511 levothyroxine users aged 70 years or older from a health insurance database were followed up for 3.8 years, and the odds ratio for fracture was 3.45 (95% CI, 3.27 to 3.65) for the highest dose group (>93 µg/d), compared to the lowest dose group (<44 µg/d). This study showed that current levothyroxine treatment was associated with a significantly increased fracture risk in a strong dose-response relationship. Since previous studies did not consider the patients' baseline osteoporosis statuses, further studies are needed to evaluate the safe levothyroxine ranges according to the osteoporosis status.
- These results agreed with the pathologic mechanisms of iatrogenic hyperthyroidism caused by excessive levothyroxine use [24]. The pathogenic mechanism that affects the bones in hyperthyroidism is based on an increase in both the number and turnover rate of bone turnover units and thus increases in osteoclast and osteoblast activity, with 50% reductions in the remodeling cycle and increased unit activation frequency. These changes lead to an uncoupling of resorption and formation, and the net result of mineralized bone losses in varying amounts depends on factors such as sex, menstrual status, thyroid disease severity, and the sum of other osteoporosis risk factors [25,26]. The result of a previous study about the interaction between iatrogenic hyperthyroidism-mediated reduced BMD and osteoporosis severity were limited. However, these 2 conditions should interact strongly with each other because they share the similar pathologic mechanism of reduced BMD. Additionally, in our study, an significant interaction between the levothyroxine dose and osteoporosis status was observed in logistic regression (p<0.001).
- Our results indicated no significant relationship between the daily levothyroxine dose and fracture risk in non-severe osteoporosis patients. When we calculated the appropriate sample size assuming 80% power, 5% alpha-error, and a 3% expected fracture incidence in unexposed group, 1492 patients were required to confirm a relative risk of 2.0. In our study, the minimum number of participants in 1 comparison was 1565 patients in the non-severe osteoporosis subgroup. Therefore, the number of participants in this study's subgroup analyses was sufficient to examine the relationship between the daily levothyroxine dose and fracture risk.
- We considered the potential occurrence of "confounding by indication," in which prognostic factors influence the treatment decisions. In our study, we evaluated the factors associated with the indications used to decide the levothyroxine doses and fracture risk factors. The AACE and ATA guidelines for hypothyroidism management suggested that patients with angina symptoms should receive half of the regular levothyroxine dose at the start of levothyroxine treatment for hypothyroidism; furthermore, the target TSH level was also higher in this population [14]. To evaluate the previous medical histories associated with angina symptoms, we analyzed covariates such as ischemic heart disease, arrhythmia, stroke, and heart failure in multiple logistic regressions. However, we did not find any associations between the fracture risk and these variables in any osteoporosis subgroup. Furthermore, we evaluated the differences in the average daily levothyroxine doses in the lowest dose groups according to the degree of osteoporosis, and no significant differences were observed in our data with ANOVA (p=0.43; average daily levothyroxine doses: 46.5 µg/d for the non-osteoporosis subgroup, 46.7 µg/d for the probable osteoporosis subgroup, and 47.2 µg/d for the highly probable osteoporosis subgroup).
- Another considerable bias was misclassification. According to the previous osteoporosis history, it was expected that patients who were included in the highly probable osteoporosis subgroup would have homogenous characteristics. Until 2011, physicians in Korea could prescribe osteoporosis-specific drugs such as bisphosphonate and raloxifene when the T-scores of the lumbar spine or femur neck were less than -3 according to dual-energy X-ray absorptiometry (DXA) because of unitary insurance coverage regulations. Therefore, a bisphosphonate or raloxifene prescription indicated that a patient's T-scores according to DXA were less than -3, consequently, the highly probable osteoporosis subgroup comprised homogenous patients with severe osteoporosis [27]. In many epidemiologic studies that used claims databases, operational definitions of osteoporosis diagnosis such as combined ICD-10 codes of osteoporosis and the application of exclusive medications for osteoporosis treatment were used [16,28]
- In clinical practice, low patient adherence is a common problem associated with levothyroxine therapy [29]. To eliminate the effects of low patient adherence, we included patients with adequate levothyroxine adherence, according to the PDC (≥80%) and the maximum daily dose criterion (<300 µg/d). PDC was developed to evaluate prescription adherence in chronic drug use situations [21]. Generally, PDC above 80% is considered appropriate for drugs used to treat chronic diseases such as hypertension and DM [20]. Additionally, we restricted our analytical cohort to patients who were prescribed levothyroxine doses <300 µg/d. Garber et al. [14] and Ain et al. [19] reported that most patients who were regularly prescribed levothyroxine doses ≥300 µg/d had low levothyroxine adherence.
- Our results should be considered in light of several limitations. First, the data in our study did not include body weight and lifestyle factors such as smoking history and alcohol use status. However, in previous epidemiologic studies, the fracture risk estimates were little affected by adjustments for body weight or lifestyle factors [30,31]. Second, our study participants included previous levothyroxine users and thus did not comprise wholly new users. In a sensitivity analysis restricted to new users (defined as not having used levothyroxine during the first 6 months of the study period), we identified the fracture risk according to the daily levothyroxine dose. These new users included 1396 patients, among whom 21 patients experienced fractures. The HRs among the new users were 1.89 (95% CI, 0.66 to 5.40), 0.58 (95% CI, 0.08 to 4.43), and 2.10 (95% CI, 0.28 to 15.61) in the ≤50 µg/d, 101 to 150 µg/d, and >150 µg/d groups, respectively, when compared to the 51 to 100 µg/d group. Given the lack of statistical power, we could not conclude the fracture risk according to the levothyroxine dose among new users. Therefore, further studies based on longer follow-up periods are recommended to confirm the association between the levothyroxine dose and fracture risk. Third, the data in our study were left-truncated because the dataset did not contain the medical records of participants before 2005. Consequently, we could not ascertain the previous levothyroxine prescription and medical history statuses before the starting point of the data. Therefore, we applied the statistical method for left-truncated data reported by Cain et al. [23]. Bias can be reduced when analyses account for left truncation, although the results are unstable when the truncated fraction is high. Additionally, this method adjusts for a just distribution of outcome events, but does not adjust for exposure. Fourth, our data did not contain records for the pharmaceutical drug preparations or the actual drug ingestion of the patients in real-life. Therefore, we could not identify the true levothyroxine intake amounts of the patients. The last limitation of this study was related to the validity of diagnoses such as fractures and hypothyroidism. Our data did not include laboratory and clinical information such as the BMD and TSH levels. To increase the validity, we developed operational definitions of fracture, hypothyroidism, and osteoporosis, which were restricted to fractures diagnosed by the department of orthopedic surgery or emergency medicine, the diagnosis of hypothyroidism with thyroid hormone replacement therapy use, and the diagnosis of osteoporosis with osteoporosis-specific drug prescriptions, respectively. Additionally, a validation study was used to compare the diagnoses derived from the medical insurance claims database in Korea with the actual diagnoses in the medical records. The overall positive predictive value of the diagnoses was 81.8% in the overall hospitalized patient population [32].
- Despite these limitations, our study has some strengths. The HIRA database was used as a data source, and accurate verification of the starting point, duration, and dosage of the drugs was available during the study period. Consequently, information bias, especially recall bias, was possibly minimal. Furthermore, our novel finding provides evidence for an association between the fracture risk and levothyroxine dosage, according to the osteoporosis status.
- In conclusion, prescribed levothyroxine doses of >150 µg/d were associated with a higher risk of fracture in elderly female patients with severe osteoporosis. While further studies are needed to establish evidence for the risk-benefit balance, physicians should be concerned about potential levothyroxine overtreatment in elderly women with histories of severe osteoporosis.
DISCUSSION
ACKNOWLEDGEMENTS
- 1. Roberts CG, Ladenson PW. Hypothyroidism. Lancet 2004;363(9411):793-803. 15016491ArticlePubMed
- 2. Choi HS, Park YJ, Kim HK, Choi SH, Lim S, Park DJ, et al. Prevalence of subclinical hypothyroidism in two population based-cohort: Ansung and KLoSHA Cohort in Korea. J Korean Thyroid Assoc 2010;3(1):32-40. (Korean)
- 3. Sheppard MC, Holder R, Franklyn JA. Levothyroxine treatment and occurrence of fracture of the hip. Arch Intern Med 2002;162(3):338-343. 11822927ArticlePubMed
- 4. Flynn RW, Bonellie SR, Jung RT, MacDonald TM, Morris AD, Leese GP. Serum thyroid-stimulating hormone concentration and morbidity from cardiovascular disease and fractures in patients on long-term thyroxine therapy. J Clin Endocrinol Metab 2010;95(1):186-193. 19906785ArticlePubMed
- 5. Lee JS, Buzkova P, Fink HA, Vu J, Carbone L, Chen Z, et al. Subclinical thyroid dysfunction and incident hip fracture in older adults. Arch Intern Med 2010;170(21):1876-1883. 21098345ArticlePubMedPMC
- 6. Robbins J. Factors altering thyroid hormone metabolism. Environ Health Perspect 1981;38: 65-70. 7238448ArticlePubMedPMC
- 7. Mooradian AD. Normal age-related changes in thyroid hormone economy. Clin Geriatr Med 1995;11(2):159-169. 7606687ArticlePubMed
- 8. Canaris GJ, Manowitz NR, Mayor G, Ridgway EC. The Colorado thyroid disease prevalence study. Arch Intern Med 2000;160(4):526-534. 10695693ArticlePubMed
- 9. Hollowell JG, Staehling NW, Flanders WD, Hannon WH, Gunter EW, Spencer CA, et al. Serum TSH, T(4), and thyroid antibodies in the United States population (1988 to 1994): National Health and Nutrition Examination Survey (NHANES III). J Clin Endocrinol Metab 2002;87(2):489-499. 11836274ArticlePubMed
- 10. Somwaru LL, Arnold AM, Joshi N, Fried LP, Cappola AR. High frequency of and factors associated with thyroid hormone over-replacement and under-replacement in men and women aged 65 and over. J Clin Endocrinol Metab 2009;94(4):1342-1345. 19126628ArticlePubMedPMC
- 11. Faber J, Galløe AM. Changes in bone mass during prolonged subclinical hyperthyroidism due to L-thyroxine treatment: a meta-analysis. Eur J Endocrinol 1994;130(4):350-356. 8162163ArticlePubMed
- 12. Uzzan B, Campos J, Cucherat M, Nony P, Boissel JP, Perret GY. Effects on bone mass of long term treatment with thyroid hormones: a meta-analysis. J Clin Endocrinol Metab 1996;81(12):4278-4289. 8954028ArticlePubMed
- 13. Turner MR, Camacho X, Fischer HD, Austin PC, Anderson GM, Rochon PA, et al. Levothyroxine dose and risk of fractures in older adults: nested case-control study. BMJ 2011;342: d2238. 21527461ArticlePubMedPMC
- 14. Garber JR, Cobin RH, Gharib H, Hennessey JV, Klein I, Mechanick JI, et al. Clinical practice guidelines for hypothyroidism in adults: cosponsored by the American Association of Clinical Endocrinologists and the American Thyroid Association. Endocr Pract 2012;18(6):988-1028. 23246686ArticlePubMed
- 15. Kwon S. Payment system reform for health care providers in Korea. Health Policy Plan 2003;18(1):84-92. 12582111ArticlePubMed
- 16. Park C, Ha YC, Jang S, Jang S, Yoon HK, Lee YK. The incidence and residual lifetime risk of osteoporosis-related fractures in Korea. J Bone Miner Metab 2011;29(6):744-751. 21644058ArticlePubMed
- 17. Lee YK, Jang S, Jang S, Lee HJ, Park C, Ha YC, et al. Mortality after vertebral fracture in Korea: analysis of the National Claim Registry. Osteoporos Int 2012;23(7):1859-1865. 22109741ArticlePubMed
- 18. Choi HJ, Shin CS, Ha YC, Jang S, Jang S, Park C, et al. Burden of osteoporosis in adults in Korea: a national health insurance database study. J Bone Miner Metab 2012;30(1):54-58. 21633927ArticlePubMed
- 19. Ain KB, Refetoff S, Fein HG, Weintraub BD. Pseudomalabsorption of levothyroxine. JAMA 1991;266(15):2118-2120. 1920700ArticlePubMed
- 20. Peterson AM, Nau DP, Cramer JA, Benner J, Gwadry-Sridhar F, Nichol M. A checklist for medication compliance and persistence studies using retrospective databases. Value Health 2007;10(1):3-12. 17261111ArticlePubMed
- 21. Benner JS, Glynn RJ, Mogun H, Neumann PJ, Weinstein MC, Avorn J. Long-term persistence in use of statin therapy in elderly patients. JAMA 2002;288(4):455-461. 12132975ArticlePubMed
- 22. Song HJ, Lee J, Kim YJ, Jung SY, Kim HJ, Choi NK, et al. β1 selectivity of β-blockers and reduced risk of fractures in elderly hypertension patients. Bone 2012;51(6):1008-1015. 22960238ArticlePubMed
- 23. Cain KC, Harlow SD, Little RJ, Nan B, Yosef M, Taffe JR, et al. Bias due to left truncation and left censoring in longitudinal studies of developmental and disease processes. Am J Epidemiol 2011;173(9):1078-1084. 21422059ArticlePubMedPMC
- 24. Ross DS, Ardisson LJ, Meskell MJ. Measurement of thyrotropin in clinical and subclinical hyperthyroidism using a new chemiluminescent assay. J Clin Endocrinol Metab 1989;69(3):684-688. 2760176ArticlePubMed
- 25. Mosekilde L, Eriksen EF, Charles P. Effects of thyroid hormones on bone and mineral metabolism. Endocrinol Metab Clin North Am 1990;19(1):35-63. 2192868ArticlePubMed
- 26. Faber J, Jensen IW, Petersen L, Nygaard B, Hegedus L, Siersbaek-Nielsen K. Normalization of serum thyrotrophin by means of radioiodine treatment in subclinical hyperthyroidism: effect on bone loss in postmenopausal women. Clin Endocrinol (Oxf) 1998;48(3):285-290. 9578817ArticlePubMed
- 27. Boelaert K. Thyroid dysfunction in the elderly. Nat Rev Endocrinol 2013;9(4):194-204. 23438834ArticlePubMed
- 28. Jang S, Park C, Jang S, Yoon HK, Shin CS, Kim DY, et al. Medical service utilization with osteoporosis. Endocrinol Metab 2010;25(4):326-339. (Korean)Article
- 29. Laurberg P, Andersen S, Bülow Pedersen I, Carle A. Hypothyroidism in the elderly: pathophysiology, diagnosis and treatment. Drugs Aging 2005;22(1):23-38. 15663347ArticlePubMed
- 30. Pasco JA, Henry MJ, Sanders KM, Kotowicz MA, Seeman E, Nicholson GC, et al. Beta-adrenergic blockers reduce the risk of fracture partly by increasing bone mineral density: Geelong Osteoporosis Study. J Bone Miner Res 2004;19(1):19-24. 14753732ArticlePubMed
- 31. Yang S, Nguyen ND, Center JR, Eisman JA, Nguyen TV. Association between beta-blocker use and fracture risk: the Dubbo Osteoporosis Epidemiology Study. Bone 2011;48(3):451-455. 21047567ArticlePubMed
- 32. Park BJ, Sung JH, Park KD, Seo SW, Kim SW. Report of the evaluation for validity of discharged diagnoses in Korean Health Insurance database. Seoul: Seoul National University; 2003. p. 19-52 (Korean)
REFERENCES
| Characteristics | Highly probable osteoporosis (%)1 | Probable osteoporosis (%)2 | Non-osteoporosis (%)3 | p-value |
|---|---|---|---|---|
| Daily levothyroxine dose (mean±SD, μg/d) | 98.2±42.2 | 101.6±44.5 | 99.0±43.5 | 0.02 |
| ≤50 | 453 (21.0) | 468 (19.2) | 1375 (21.0) | |
| 51-100 | 1193 (55.3) | 1306 (53.6) | 3598 (54.8) | |
| 101-150 | 328 (15.2) | 404 (16.6) | 942 (14.4) | |
| >150 | 183 (8.5) | 259 (10.6) | 646 (9.9) | |
| Age (mean±SD) | 71.0±4.9 | 70.1±4.6 | 70.8±5.2 | <0.01 |
| 65-69 | 987 (45.8) | 1306 (53.6) | 3217 (49.0) | |
| 70-74 | 676 (31.3) | 699 (28.7) | 1838 (28.0) | |
| 75-79 | 360 (16.7) | 331 (13.6) | 1035 (15.8) | |
| ≥80 | 134 (6.2) | 101 (4.1) | 471 (7.2) | |
| Comorbidity | ||||
| Malignancy | 140 (6.5) | 138 (5.7) | 361 (5.5) | 0.23 |
| Diabetes Mellitus | 829 (38.4) | 965 (39.6) | 2570 (39.2) | 0.72 |
| Hypoparathyroidism | 24 (1.1) | 60 (2.5) | 32 (0.5) | <0.01 |
| Cushing syndrome | 20 (0.9) | 7 (0.3) | 20 (0.3) | <0.01 |
| Adrenal disorder | 30 (1.4) | 10 (0.4) | 28 (0.4) | <0.01 |
| COPD | 444 (20.6) | 479 (19.7) | 1087 (16.6) | <0.01 |
| Asthma | 591 (27.4) | 659 (27.0) | 1488 (22.7) | <0.01 |
| Chronic liver disease | 402 (18.6) | 527 (21.6) | 1169 (17.8) | <0.01 |
| Chronic kidney disease | 32 (1.5) | 41 (1.7) | 207 (3.2) | <0.01 |
| Heart failure | 150 (7.0) | 168 (6.9) | 550 (8.4) | 0.02 |
| Ischemic heart disease | 508 (23.6) | 566 (23.3) | 1473 (22.5) | 0.50 |
| Arrhythmia | 174 (8.1) | 175 (7.2) | 557 (8.5) | 0.13 |
| Dementia | 77 (3.6) | 64 (2.6) | 184 (2.8) | 0.12 |
| Stroke | 263 (12.2) | 277 (11.4) | 716 (10.9) | 0.26 |
| Epilepsy | 54 (2.5) | 51 (2.1) | 134 (2.0) | 0.43 |
| Charlson comorbidity score (mean±SD) | 2.41±1.95 | 2.31±1.91 | 2.14±1.94 | <0.01 |
| 0 | 333 (15.4) | 383 (15.7) | 1236 (18.8) | |
| 1 | 454 (21.1) | 556 (22.8) | 1712 (26.1) | |
| 2 | 462 (21.4) | 550 (22.6) | 1376 (21.0) | |
| 3 | 398 (18.5) | 413 (17.0) | 945 (14.4) | |
| ≥4 | 510 (23.6) | 535 (22.0) | 1292 (19.7) | |
| Co-medication | ||||
| Glucocorticoid | 1462 (67.8) | 1593 (65.4) | 3559 (54.2) | <0.01 |
| Anti-coagulants | 47 (2.2) | 49 (2.0) | 170 (2.6) | 0.22 |
| Anti-epileptics | 408 (18.9) | 347 (14.2) | 7665 (11.7) | <0.01 |
| Anti-depressants | 546 (25.3) | 563 (23.1) | 1337 (20.4) | <0.01 |
| Benzodiazepines | 1538 (71.3) | 1675 (68.7) | 4114 (62.7) | <0.01 |
| Proton pump inhibitor | 279 (12.9) | 299 (12.3) | 575 (8.8) | <0.01 |
| Thiazolidinedion | 69 (3.2) | 73 (3.0) | 229 (3.5) | 0.48 |
| No of health service use (mean±SD) | 38.8±18.7 | 37.8±17.8 | 30.9±15.4 | <0.01 |
| 1-19 | 270 (12.5) | 318 (13.1) | 1531 (23.3) | |
| 20-39 | 965 (44.7) | 1150 (47.2) | 3449 (52.6) | |
| 40-59 | 640 (29.7) | 680 (27.9) | 1265 (19.3) | |
| ≥60 | 282 (13.1) | 289 (11.9) | 316 (4.8) | |
| Total | 2157 (100.0) | 2437 (100.0) | 6561 (100.0) |
COPD, chronic obstructive pulmonary disease.
1 Included patients who were diagnosed with osteoporosis and were prescribed bisphosphonate or raloxifene.
2 Included patients who were diagnosed with osteoporosis, but were not prescribed bisphosphonate or raloxifene.
3 Included patients who were neither diagnosed with osteoporosis nor prescribed bisphosphonate.
| Daily levothyroxine dose (μg/d) | No. of participants (%) | No. of events | Incidence rate (/1,000 PY) | cHR (95% CI) | aHR1 (95% CI) |
|---|---|---|---|---|---|
| Osteoporosis2 | |||||
| ≤50 | 921 (20.1) | 46 | 42.4 | 1.22 (0.86, 1.75) | 1.16 (0.80, 1.66) |
| 51-100 | 2499 (54.4) | 115 | 36.0 | Reference | Reference |
| 101-150 | 732 (15.9) | 37 | 37.9 | 1.09 (0.75, 1.58) | 1.16 (0.80, 1.69) |
| >150 | 442 (9.6) | 29 | 50.7 | 1.49 (0.99, 2.24) | 1.56 (1.03, 2.37) |
| 4594 (100.0) | 227 | 39.0 | |||
| Highly probable osteoporosis3 | |||||
| ≤50 | 453 (21.0) | 22 | 41.9 | 0.97 (0.60, 1.57) | 0.93 (0.55, 1.56) |
| 51-100 | 1193 (55.3) | 67 | 44.3 | Reference | Reference |
| 101-150 | 328 (15.2) | 18 | 41.1 | 0.91 (0.54, 1.54) | 0.92 (0.54, 1.58) |
| >150 | 183 (8.5) | 19 | 81.9 | 1.85 (1.11, 3.07) | 1.93 (1.14, 3.26) |
| 2157 (100.0) | 126 | 46.5 | |||
| Probable osteoporosis4 | |||||
| ≤50 | 468 (19.2) | 24 | 42.9 | 1.52 (0.93, 2.48) | 1.43 (0.84, 2.42) |
| 51-100 | 1306 (53.6) | 48 | 28.6 | Reference | Reference |
| 101-150 | 404 (16.6) | 19 | 35.3 | 1.23 (0.72, 2.09) | 1.60 (0.90, 2.82) |
| >150 | 259 (10.6) | 10 | 29.4 | 1.03 (0.52, 2.03) | 1.25 (0.62, 2.55) |
| 2437 (100.0) | 101 | 32.4 | |||
| Non-osteoporosis5 | |||||
| ≤50 | 1375 (21.0) | 52 | 31.7 | 1.07 (0.78, 1.47) | 1.05 (0.75, 1.47) |
| 51-100 | 3598 (54.8) | 138 | 29.9 | Reference | Reference |
| 101-150 | 942 (14.4) | 44 | 35.4 | 1.18 (0.84, 1.66) | 1.10 (0.78, 1.56) |
| >150 | 646 (9.9) | 22 | 26.2 | 0.88 (0.56, 1.37) | 0.84 (0.52, 1.35) |
| 6561 (100.0) | 256 | 30.7 | |||
| All elderly women in our dataset | |||||
| 2 533 289 | 175 783 | 46.3 |
PY, person-year; cHR, crude hazard ratio; CI, confidence intervals; aHR, adjusted hazard ratio.
1 Adjusted for age, comorbidities, comedications, Charlson comorbidity score, and number of health service uses.
2 Included patients who were diagnosed with osteoporosis, regardless of bisphosphonate or raloxifene prescription status.
3 Included patients who were diagnosed with osteoporosis and were prescribed bisphosphonate or raloxifene.
4 Included patients who were diagnosed with osteoporosis, but were not prescribed bisphosphonate or raloxifene.
5 Included patients who were neither diagnosed with osteoporosis nor prescribed bisphosphonate.
Figure & Data
References
Citations

- Diagnosis and therapeutic approach to bone health in patients with hypopituitarism
Justyna Kuliczkowska-Płaksej, Aleksandra Zdrojowy-Wełna, Aleksandra Jawiarczyk-Przybyłowska, Łukasz Gojny, Marek Bolanowski
Reviews in Endocrine and Metabolic Disorders.2024;[Epub] CrossRef - Evaluation and Management of Bone Health in Patients with Thyroid Diseases: A Position Statement of the Korean Thyroid Association
A Ram Hong, Ho-Cheol Kang
Endocrinology and Metabolism.2023; 38(2): 175. CrossRef - Refractory Hypothyroidism: Unraveling the Complexities of Diagnosis and Management
Juan Eduardo Quiroz-Aldave, Marcio José Concepción-Zavaleta, María del Carmen Durand-Vásquez, Luis Alberto Concepción-Urteaga, Elman Rolando Gamarra-Osorio, Jacsel Suárez-Rojas, Luciana del Pilar Rafael-Robles, José Paz-Ibarra, Alejandro Román-González
Endocrine Practice.2023; 29(12): 1007. CrossRef - Assessing the cardiovascular effects of levothyroxine use in an ageing United Kingdom population (ACEL-UK) protocol: a cohort and target trial emulation study
Mia Holley, Salman Razvi, Rosie Dew, Ian Maxwell, Scott Wilkes
Thyroid Research.2023;[Epub] CrossRef - Understanding Worry About Risks Associated With Thyroid Hormone Therapy: A National Survey of Endocrinologists, Family Physicians, and Geriatricians
Kimi Shah, David Reyes-Gastelum, Brittany L. Gay, Maria Papaleontiou
Endocrine Practice.2022; 28(1): 25. CrossRef - Evaluation and Management of Bone Health in Patients with Thyroid Diseases: a Position Statement from the Korean Thyroid Association
A Ram Hong, Hwa Young Ahn, Bu Kyung Kim, Seong Hee Ahn, So Young Park, Min-Hee Kim, Jeongmin Lee, Sun Wook Cho, Ho-Cheol Kang
International Journal of Thyroidology.2022; 15(1): 1. CrossRef - Levothyroxine Therapy in Elderly Patients With Hypothyroidism
Grigoris Effraimidis, Torquil Watt, Ulla Feldt-Rasmussen
Frontiers in Endocrinology.2021;[Epub] CrossRef - Systemic medicines taken by adult special care dental patients and implications for the management of their care
Nicholas Ransford, Ben Marnell, Christine Randall, Clare Yates, Gillian Howie
British Dental Journal.2021; 231(1): 33. CrossRef - The Influence of Thyroid Pathology on Osteoporosis and Fracture Risk: A Review
Dragos Apostu, Ondine Lucaciu, Daniel Oltean-Dan, Alexandru-Dorin Mureșan, Cristina Moisescu-Pop, Andrei Maxim, Horea Benea
Diagnostics.2020; 10(3): 149. CrossRef - Comparison of the efficacy between once-monthly oral ibandronate and risedronate among Korean women with osteoporosis: a nationwide population-based study
Dong Ryul Lee, Jungun Lee
Osteoporosis International.2019; 30(3): 659. CrossRef - The effect of thyroid functions on osteopenia of prematurity in preterm infants
Ufuk Çakır, Cuneyt Tayman
Journal of Pediatric Endocrinology and Metabolism.2019; 32(1): 65. CrossRef - Understanding hypothyroidism in Unani medicine
Md. Anzar Alam, Mohd Aleemuddin Quamri, Ghulamuddin Sofi, Barkati Md. Tarique
Journal of Integrative Medicine.2019; 17(6): 387. CrossRef - Team Approach: Multidisciplinary Treatment of Hip Fractures in Elderly Patients
Wender Figved, Marius Myrstad, Ingvild Saltvedt, Merete Finjarn, Liv Marie Flaten Odland, Frede Frihagen
JBJS Reviews.2019; 7(6): e6. CrossRef - Effect of sodium levothyroxine on histomorphometry, histopathology, histochemistry, and immunohistochemistry of articular cartilage in female mice
Simin Fazelipour, Minoo Shafii, Mahsa Hadipour Jahromi, Zahra Tootian, Mohammad Taghi Sheibani, Hassan Morovvati, Marzieh Minaei, Anahita Shahriari, Pooneh Koochaki, Safora Karimi
Comparative Clinical Pathology.2018; 27(1): 45. CrossRef - High Prevalence of Radiological Vertebral Fractures in Women on Thyroid-Stimulating Hormone–Suppressive Therapy for Thyroid Carcinoma
Gherardo Mazziotti, Anna Maria Formenti, Stefano Frara, Roberto Olivetti, Giuseppe Banfi, Maurizio Memo, Roberto Maroldi, Raffaele Giubbini, Andrea Giustina
The Journal of Clinical Endocrinology & Metabolism.2018; 103(3): 956. CrossRef - Subclinical thyrotoxicosis and bone
M. Doga, A.M. Formenti, S. Frara, M. Memo, A. Giustina, G. Mazziotti
Current Opinion in Endocrine and Metabolic Researc.2018;[Epub] CrossRef - Effects of Abnormal Thyroid Function Status to Bone Metabolism
Hwa Young Ahn
International Journal of Thyroidology.2018; 11(1): 21. CrossRef - RETRACTED ARTICLE: The relationship between subclinical thyroid dysfunction and the risk of fracture or low bone mineral density: a systematic review and meta-analysis of cohort studies
Ruifei Yang, Liang Yao, Yuan Fang, Jing Sun, Tiankang Guo, Kehu Yang, Limin Tian
Journal of Bone and Mineral Metabolism.2018; 36(2): 209. CrossRef - MANAGEMENT OF ENDOCRINE DISEASE: l-Thyroxine replacement therapy in the frail elderly: a challenge in clinical practice
R M Ruggeri, F Trimarchi, B Biondi
European Journal of Endocrinology.2017; 177(4): R199. CrossRef - THE IMPACT OF AGE IN THE MANAGEMENT OF HYPOTHYROIDISM: RESULTS OF A NATIONWIDE SURVEY
Maria Papaleontiou, Brittany L. Gay, Nazanene H. Esfandiari, Sarah T. Hawley, Megan R. Haymart
Endocrine Practice.2016; 22(6): 708. CrossRef - TSH Suppression after Differentiated Thyroid Cancer Surgery and Osteoporosis
Kyoung Sik Park
Korean Journal of Endocrine Surgery.2016; 16(1): 1. CrossRef - TSH Suppression after Differentiated Thyroid Cancer Surgery and Osteoporosis
Kyoung Sik Park
Korean Journal of Endocrine Surgery.2016; 16(1): 1. CrossRef - Role of Thyroid Hormones in Skeletal Development and Bone Maintenance
J. H. Duncan Bassett, Graham R. Williams
Endocrine Reviews.2016; 37(2): 135. CrossRef
 KSPM
KSPM

 PubReader
PubReader ePub Link
ePub Link Cite
Cite