Articles
- Page Path
- HOME > J Prev Med Public Health > Volume 45(4); 2012 > Article
-
Original Article
Zolpidem Use and Risk of Fracture in Elderly Insomnia Patients - Dong-Yoon Kang1, Soyoung Park1, Chul-Woo Rhee1, Ye-Jee Kim1, Nam-Kyong Choi2, Joongyub Lee2, Byung-Joo Park1,2
-
Journal of Preventive Medicine and Public Health 2012;45(4):219-226.
DOI: https://doi.org/10.3961/jpmph.2012.45.4.219
Published online: July 31, 2012
1Department of Preventive Medicine, Seoul National University College of Medicine, Seoul, Korea.
2Medical Research Collaborating Center, Seoul National University Hospital, Seoul, Korea.
- Corresponding author: Byung-Joo Park, MD, PhD. 103 Daehak-ro, Jongno-gu, Seoul 110-799, Korea. Tel: +82-2-740-8325, Fax: +82-2-747-4830, bjpark@snu.ac.kr
• Received: August 31, 2011 • Accepted: November 3, 2011
Copyright © 2012 The Korean Society for Preventive Medicine
This is an Open Access article distributed under the terms of the Creative Commons Attribution Non-Commercial License (http://creativecommons.org/licenses/by-nc/3.0/) which permits unrestricted non-commercial use, distribution, and reproduction in any medium, provided the original work is properly cited.
ABSTRACT
-
Objectives
- To evaluate the risk of fractures related with zolpidem in elderly insomnia patients.
-
Methods
- Health claims data on the entire South Korean elderly population from January 2005 to June 2006 were extracted from the Health Insurance Review and Assessment Service database. We applied a case-crossover design. Cases were defined as insomnia patients who had a fracture diagnosis. We set the hazard period of 1 day length prior to the fracture date and four control periods of the same length at 5, 10, 15, and 20 weeks prior to the fracture date. Time independent confounding factors such as age, gender, lifestyle, cognitive function level, mobility, socioeconomic status, residential environment, and comorbidity could be controlled using the casecrossover design. Time dependent confounding factors, especially co-medication of patients during the study period, were adjusted by conditional logistic regression analysis. The odds ratios and their 95% confidence intervals (CIs) were estimated for the risk of fracture related to zolpidem.
-
Results
- One thousand five hundred and eight cases of fracture were detected in insomnia patients during the study period. In our data, the use of zolpidem increased the risk of fracture significantly (adjusted odds ratio [aOR], 1.72; 95% CI, 1.37 to 2.16). However, the association between benzodiazepine hypnotics and the risk of fracture was not statistically significant (aOR, 1.00; 95% CI, 0.83 to 1.21). Likewise, the results were not statistically significant in stratified analysis with each benzodiazepine generic subgroup.
-
Conclusions
- Zolpidem could increase the risk of fracture in elderly insomnia patients. Therefore zolpidem should be prescribed carefully and the elderly should be provided with sufficient patient education.
- The prevalence of insomnia increases with age. The trend toward emphasizing quality of life has made sound sleep an important issue. However, the changing social structure can cause sleep disturbance. In Korea, more than 20% of the population suffers from insomnia including 35% among those 65 years old and above [1,2]. As Korean society progresses from an aging society towards an aged society, the prevalence rate of insomnia and demand for insomnia control will continue to increase.
- Cognitive-behavioral therapy is the first-line treatment for insomnia. However, medication is also a major treatment option offered in clinics [3,4]. Higher insomnia prevalence leads to higher hypnotic prescription and higher rates of adverse drug reactions. This could have serious results, especially in the elderly, because of their reduced drug metabolism. Even after waking up, drowsiness, dizziness, decreased alertness and concentration, and lack of coordination could endure. These residual hypnotic effects may lead to lethal results such as a fracture [5].
- Nowadays, benzodiazepines and short acting non-benzodiazepine hypnotics are the main medication for controling insomnia. Even some tricyclic antidepressants and antihistamines are used to induce sleep [6-8]. Since 1960s, the benzodiazepines were established as the major drug for treating insomnia due to their marked stability compared to formerly popular hypnotics. But, after the 1980s, the development of addiction with long-term use and accidents due to residual hypnotic effects made this drug a serious social issue [9,10]. New hypnotics, such as zolpidem, zopiclone, zaleplon, and eszopiclone, the so-called "Z-drugs," appeared in the 1990s and rapidly became popular because of their much better safety record [7]. Zolpidem, a representative drug of these new hypnotics, is the most popular medicine for treating insomnia in the US at present. It is known for low tolerance, a quick induction time usually within 15 minutes, and a short half-life of two to three hours, which reduces the residual 'hangover' effects, such as sleepiness and impaired psychomotor and cognitive function, after nighttime administration that may persist into the next day [11-13]. Therefore, zolpidem was expected to be a safer alternative to the benzodiazepines, and many studies have supported its effect and safety thenceforth [14-18].
- However, zolpidem could not eliminate the possibility of fracture completely [19]. Many consistent reports have claimed a fall or fracture occurred due to somnambulism (though in very low frequency) [20-23], and questions still remain about whether zolpidem is safer than the existing benzodiazepine drugs as far as risk of fracture [24]. In elderly, it was reported much serious central nervous system effects such as lack of consciousness. And risk of fracture due to fall was not much lower or even higher than the existing benzodiazepine hypnotics [25-28]. Thereupon, this study aimed to examine the risk of fracture associated with zolpidem use in elderly insomnia patients.
INTRODUCTION
- Data Source
- Data from the Korean Health Insurance Review and Assessment Service (HIRA) database, which contains all claims for the medication and medical procedures of approximately 50 million Koreans, between January 2005 and June 2006 were used. The database included the age, sex, prescription, and diagnosis information. The prescription data included the brand name and generic name of the drug, the date of the prescription, the amount of medication, the duration of medication, etc. Diagnoses were coded according to the International Statistical Classification of Diseases and Related Health Problems, 10th revision (ICD-10). The institutional review board of Seoul National University Hospital and Seoul National University College of Medicine approved the study protocol (protocol ID: E-1105-007-360).
- Definition of Cases
- This study targeted elderly insomnia patients who visited medical clinics anywhere in South Korea for fracture as the main diagnosis from July 1, 2005 to June 30, 2006. The targeted age for the elderly was 65 years and older at the time the fracture occurred. Insomnia patients are defined as patients who were mainly or partly diagnosed with insomnia (ICD-10: F510, G470) and who were prescribed sleeping pills more than once. Among these patients, our main research targets were people who were diagnosed with fracture (ICD-10: S02, S12, S22, S32, S42, S52, S62, S72, S82, S92, T02) and visited the emergency room or were treated with orthopedics. And the first day the patient was examined was defined as the day that fracture occurred. Once fracture occurred, when a traffic accident followed (ICD-10: V00-09) or when stroke (ICD-10: 160-64) occurred during the 180 days prior to the fracture of an individual patient, then that patient was excluded from the research sample (Figure 1).
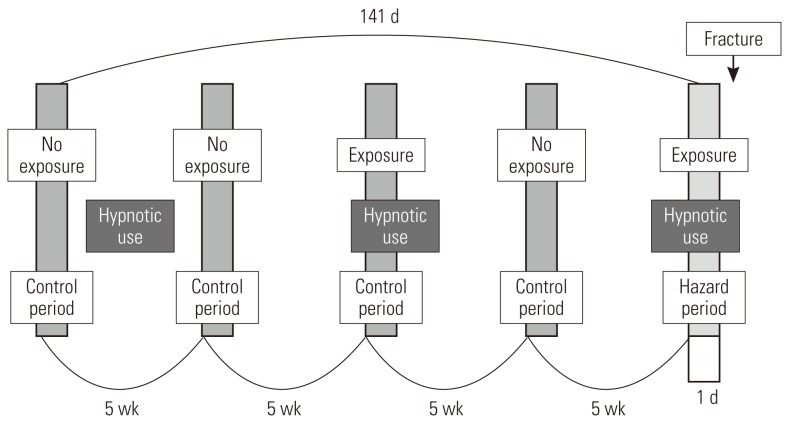
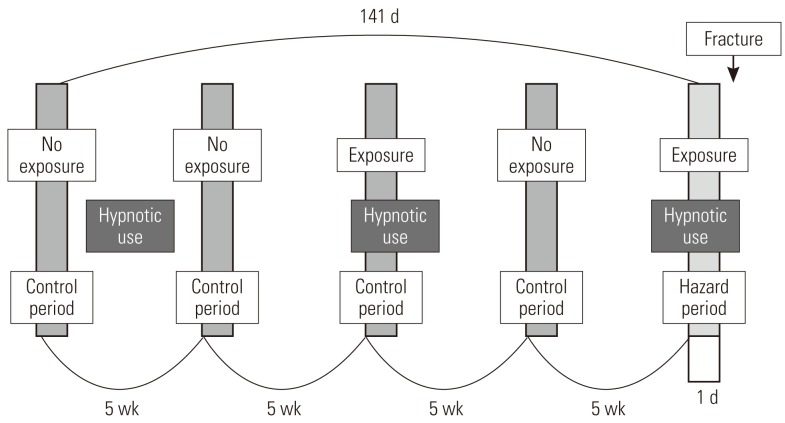
- Study Design
- This study used a case-crossover design, and Figure 2 shows the schematization of the study. For each patient, one day right before the fracture occurred was established as the hazard period, and one day before each of 5 weeks, 10 weeks, 15 weeks, and 20 weeks from the hazard period is established as the control period, setting pairs as a ratio of 1:4 [29-31]. Fracture, by its nature, is not very likely to occur again once it occurs; thus we limited the control period to the period before the fracture occurred. It is known from existing research that a short-term harmful reaction such as a hangover usually does not occur for more than a day; thus we defined the length of the hazard period as one day before the fracture [11,19,32].
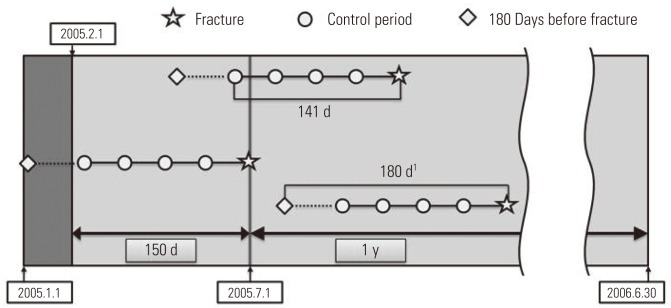
- Control of Study Period
- For the purpose of analysis, it was imperative that we confirmed the drug exposure information up to 141 days prior to the day the patient had a fracture, and we only targeted the cases that occurred in a specific year (from July 1, 2005 to June 30, 2006). We excluded patients from analysis targets who experienced a fracture before July 1, 2005, because we could not secure enough drug exposure information during the control period. In order to obtain information on the drugs that a patient took in a particular month, the data on fees charged in the previous month was required. So we ensured that the earliest control period of the patient did not earlier than in February of 2005. For example, if there is a patient who was prescribed a medicine called 'A' for 30 days on December 31, 2004, with only the charge data of January of 2005, it could appear as if the patient was not exposed to 'A' even though he or she was exposed until January 30, 2005. In particular, if the prescribed period was long, this problem could become worse, but in the case of the sleeping pill, which was the target of our research, every prescription period was less than 30 days, so we did not include in our research period the month that did not cover the hospital statement data up to 30 days and so forth. Therefore, in January of 2005, without establishing the control period and by collecting only the drug prescription data, we obtained the necessary data for a control period of February, 2005 (Figure 3).
- Exposure Assessment
- We searched every drug prescribed in Korea within the study period according to HIRA data. With the prescription data, we calculated the period of use of these drugs and the patient's exposure to zolpidem and other drugs. To adjust for irregularity of hypnotic use, we defined the period of exposure by multiplying 1.2 by the prescribed period. Considering the characteristics of sleeping pills (for example, it must be taken right before going to bed), a visit to the medical clinic due to side effects would generally take place the day after taking the drug. Thus, the very day of issuing the prescription was excluded from the exposure period. As a rule, hypnotics are prescribed for one ingredient; thus there is no reason why it should be included as a confounding variable for the analysis model of other hypnotics, but in actuality, there are cases where the clinics take doses by combining them. In these cases, other kinds of hypnotics were considered to be among the many drugs that could affect fracture, so we could adjust its effect in conditional logistic regression analysis.
- Adjusting for Other Medications
- Besides hypnotics, many kinds of drug are known from published studies to increase the risk of fall in elderly. These include the antipsychotics, antidepressants, antiepileptics, anxiolytics, analgesics, antiarrhythmic agents, diuretics, vasodilators, anticholinergics, anti-hypertensives and so on [33-37]. We collected the prescription data of these drugs from each patient and confirmed whether there was any exposure during the hazard period and control period. To create the final analysis model, we had to confirm which drug use was correlated with zolpidem exposure and had an association with fracture. In a chi-squared test of zolpidem exposure and other drug use, significant relevance was found in every drug (p<0.01). In conditional logistic regression analysis of each of the other drugs that may affect the risk of fracture in elder people, antidepressants, anxiolytics, diuretics, alpha blockers, beta blockers, and vasodilators did not have a significant effect on fracture in our study data. Thus we excluded them from the final analysis model.
- Statistical Analysis
- By adjusting for the rest of the drugs, such as antipsychotics, calcium-channel blockers, anticholinergics, antiepileptics, and analgesics, which may be confounders, we created a final analysis model that could analyze the risk ratio of fracture based on whether or not a patient was exposed to zolpidem. To compare the risk of fracture by zolpidem exposure, we generated other conditional logistic regression models that focused on benzodiazepines. Exposure to the entire group of benzodiazepine hypnotics and its generic sugroups, triazolam, lorazepam, flurazepam, flunitrazepam, midazolam, and brotizolam, were analyzed for their risk of fracture in elder insomnia patients. All analyses were done with SAS version 9.2 (SAS Inc., Cary, NC, USA).
METHODS
- One thousand five hundred and eight people were included as subjects in the final analysis through the HIRA database. Their age, sex, and comorbidities are shown in Table 1. Only the diseases diagnosed before the fracture were accepted as comorbidities. During all hazard and control periods, 431 patients were exposed to zolpidem and 703 patients were exposed to benzodiazepine more than once. The fracture of the femur (ICD-10: S72) was the most common, and there was no association between a particular fracture site and hypnotic type.
- From these 1508 subjects, after establishing one hazard period and 4 control periods, we applied conditional logistic regression analysis. The reaction risk level of fracture occurrence due to exposure to zolpidem was 1.84 and the 95% confidence interval (CI) was 1.47 to 2.30. After adjusting for the effect of other drugs that can increase the risk of fall or fracture, the reaction risk level was 1.72 and 95% CI was 1.37 to 2.16, which was also statistically significant. The reaction risk level of fracture caused by benzodiazepines (calculated using an identical method as above) was 1.12 (95% CI, 0.93 to 1.34), and after adjusting for exposure to other drugs, the reaction risk level was 1.00 (95% CI, 0.83 to 1.21), all of which were not statistically significant. The above mentioned results, even when we analyzed them by classifying drugs such as triazolam, lorazepam, flurazepam, flunitrazepam, midazolam, and brotizolam according to each ingredient, none of them had significant effects on fracture, and the same results were found even after we adjusted for exposure to other drugs. Table 2 demonstrates the results of having analyzed the risk of fracture according to the various kinds of hypnotics prescribed to elderly insomnia patients.
- We performed sensitivity analyses to calculate the risk of fracture after subdividing study subjects according to their age and gender. Zolpidem exposure increases the risk of fracture in every age group and gender over 65 years. However, the risk increased by benzodiazepine exposure was not statistically significant in stratification analysis. Table 3 demonstrates the risk of fracture stratified by age and gender.
RESULTS
- "Good" hypnotics are supposed to induce sleep quickly, have a great enough duration but low residual hypnotic effect, maintain sleep architecture, minimize tolerance, and dependence. Through many studies in the past, zolpidem was acknowledged to be a "better" sleeping pill compared to the benzodiazepine series that were being widely used at the time. In particular, it was expected that harmful occurrences, such as a fall due to the residual hypnotic effect of hypnotics, would occur much less among the elderly [38,39]. However, in reality, several studies have claimed that there are not so many differences in terms of fracture or fall [19,24,25,28], and the large-scale case-control study done by Wang et al. [27], which used charge data, showed that the risk of fracture due to prescribing zolpidem to elder patients is as close to twice as high, and this is even higher than benzodiazepine series sleeping pills which is 1.5 times as high. Even in our study, it was found that the risk of fracture was more than 1.7 times higher when exposed to zolpidem, and the risk of fracture when exposed to benzodiazepine series, which are known to increase the risk of fracture, was rather insignificant.
- Biologically, zolpidem is known to have less risk of a residual hypnotic effect the day after exposure than benzodiazepines [11-13]. When the biological theory and epidemiological result are not matched, the research should examine with more a careful approach. For example, because benzodiazepines have a long half-life, and its hangover is much worse, leading to fewer activities the next day, thus, incidents like falls could have occurred less often. Also, there was a possibility that the risk of fracture increased for other reasons than the residual hypnotic effect of zolpidem. There have been consistent reports, although low in frequency, that claimed continuous somnambulism such as driving or sleepwalking with zolpidem [20-23]. However, we were not able to confirm that in the present study because our data have no information about the subject's activity or cause of fracture. In addition, compared to zolpidem, benzodiazepines are used for purposes other than their sleep-inducing effect such as their sedative effect, and they are taken relatively consistently [24]. In this consistent using case, because the number of patients exposed to both the hazard period and control period increases, it could have happened that the risk of fracture of benzodiazepines was not assessed appropriately [29,30].
- Elderly insomnia patients usually have comorbidities and take several medications. In addition, the event of fracture could be more greatly affected by the outside environment such as living habits more than pathological progress. Therefore, we should consider many confounding variables for our analysis. The strength of this study is that we controlled for the effects of these confounding variables to the maximum degree. Within the observation period of 141 days, the possibility of varying confounding factors, including lifestyle (e.g., drinking and smoking), obesity, cognitive function level, strength of grip, mobility, socioeconomic status, and residential environment among the elderly above 65 years old, was very low. In our study, patients matched themselves as the control group; therefore, these variables were possible to control through the design of this study. Furthermore, exposure to other drugs, which continuously changed the confounding variables, could be adjusted for by using prescription data.
- For the elderly, the most powerful risk factor of fracture is age [34]. In our study, the previous 5 months of each patient became the control group, and the age distribution of the patient group and control group is identical. Through this, we controlled for the effect that age has on fracture, but when we compared the risk different hypnotics have on patients, it was discovered that if the drugs (and their ingredients) prescribed were noticeably different by age then it could distort the results. In order to assess whether there were any changes according to age, we classified the subject patients into 5-year intervals, and we calculated the frequency of prescription of zolpidem and benzodiazepine series sleeping pills as well as the reaction risk level of fracture. As a result, from the patients (those people who were subjects of this research) who were 65 years old and above, we were unable to find the difference between the prescription and the reaction risk to be significant.
- Out of the many adverse effects of hypnotics, fracture from falling is a serious harmful reaction, but due to its less frequent occurrence, it is not easy to prove a drug is a hazard. In this research, using large-scale administrative data, it was possible to secure a large enough number of subjects to statistically evaluate the risk of fracture due to exposure to zolpidem. However, since this study utilized only the fee data, the measurement of the result variable and exposure was done indirectly, and this study can be criticized for lacking the process to confirm the validity of these variable values.
- Even if the patients were prescribed sleeping pills (according to the face-value of the prescription), we cannot confirm whether the patient actually took the pills. Even in other studies that required confirming exposure to the hypnotics using prescription data, there were many attempts to revise these differences, but those studies were unable to suggest any clear methods [9,27,40]. In the present study, we judged that the method in which the risk of exposure in proportion to the prescription period was the most rational method; thus we hypothesized that there was exposure during the period where we multiplied 1.2 by the prescription period. Using this method, we tried to minimize the differences between prescription due to irregularity of zolpidem dosage and actual exposure in this research. However, in this circumstance, even when the patient did not take the sleeping pill, the result could speak as if the patient was exposed to the risk. There is a high possibility that this circumstance could occur during the control period in many cases, and as a result, there is also a possibility that the level of hazard could have appeared lower than the actuality.
- By prescription data alone, even if fracture was caused by hangover, somnambulism, or other symptoms did not caused by hypnotics, fracture used as the result variable cannot distinguish among these. In this research, in order to eliminate fracture caused by reasons other than the hypnotics as much as possible, we decided to exclude cases where they were accompanied by traffic accidents or cases where stroke occurred within 6 months since the fracture occurred in the past, but there was still a possibility that it was a fracture not caused by a harmful reaction of hypnotics, and this cannot be overlooked.
- Although we assumed that the life style factor, since it is considered not to change too much as time passes, could be controlled through matching, this is not an absolute truth. For example, even if a decrease in bone density caused by a chronic drinking habit may be controlled through matching, for a person whose drinking habit is in the form of intermittent and binge drinking, occurrence of fracture due to fall are not possible to control.
- Clinical trials assesses the safety of a new drug before marketing are performed by selecting research subjects who are few in numbers, relatively young, who had few comorbidities and co-medications, and who have a low risk of fall and fracture. For these reasons, it is difficult to confirm the causality of serious harmful instances that occurs rarely or confirm the safety in the elderly population, which is a vulnerable group. The present study was significant because we analyzed serious harmful reactions (although they occurred relatively rarely) without conflicts (e.g., ethical issues) by using large-scale administrative data, from an older population, which is a difficult group to study as direct research subjects.
- In conclusion, it was found that zolpidem, which was expected to have few cases of side effects such as fracture, among older subjects, increases the risk of fracture by 1.7 times, and there is a lack of evidence that can claim to have a lower risk of fracture compared to existing benzodiazepine series hypnotics. Therefore, when prescribing zolpidem as sleeping pills to older insomnia patients, it is necessary to be aware of this risk, and the patient should be warned and educated.
DISCUSSION
- 1. Ohayon MM, Hong SC. Prevalence of insomnia and associated factors in South Korea. J Psychosom Res 2002;53(1):593-600. 12127177ArticlePubMed
- 2. Cho YW, Shin WC, Yun CH, Hong SB, Kim J, Earley CJ. Epidemiology of insomnia in Korean adults: prevalence and associated factors. J Clin Neurol 2009;5(1):20-23. 19513329ArticlePubMedPMC
- 3. Jacobs GD, Pace-Schott EF, Stickgold R, Otto MW. Cognitive behavior therapy and pharmacotherapy for insomnia: a randomized controlled trial and direct comparison. Arch Intern Med 2004;164(17):1888-1896. 15451764ArticlePubMed
- 4. Korean NeuroPsychiatric Association. Textbook of neuropsychiatry. 2005. 2nd ed. Seoul: Jungangmunhwa Co.; p. 670-682 (Korean)
- 5. Stone KL, Ensrud KE, Ancoli-Israel S. Sleep, insomnia and falls in elderly patients. Sleep Med 2008;9(Suppl 1):S18-S22. 18929314ArticlePubMed
- 6. Maczaj M. Pharmacological treatment of insomnia. Drugs 1993;45(1):44-55. 7680984ArticlePubMed
- 7. Richey SM, Krystal AD. Pharmacological advances in the treatment of insomnia. Curr Pharm Des 2011;17(15):1471-1475. 21476952ArticlePubMed
- 8. Lenhart SE, Buysse DJ. Treatment of insomnia in hospitalized patients. Ann Pharmacother 2001;35(11):1449-1457. 11724098ArticlePubMed
- 9. Gustavsen I, Bramness JG, Skurtveit S, Engeland A, Neutel I, Morland J. Road traffic accident risk related to prescriptions of the hypnotics zopiclone, zolpidem, flunitrazepam and nitrazepam. Sleep Med 2008;9(8):818-822. 18226959ArticlePubMed
- 10. King MB. Is there still a role for benzodiazepines in general practice? Br J Gen Pract 1992;42(358):202-205. 1389432PubMedPMC
- 11. Langtry HD, Benfield P. Zolpidem: a review of its pharmacodynamic and pharmacokinetic properties and therapeutic potential. Drugs 1990;40(2):291-313. 2226217ArticlePubMed
- 12. Hoehns JD, Perry PJ. Zolpidem: a nonbenzodiazepine hypnotic for treatment of insomnia. Clin Pharm 1993;12(11):814-828. 8275648PubMed
- 13. Bogan RK. Treatment options for insomnia: pharmacodynamics of zolpidem extended-release to benefit next-day performance. Postgrad Med 2008;120(3):161-171. 18824834ArticlePubMed
- 14. Hajak G, Bandelow B. Safety and tolerance of zolpidem in the treatment of disturbed sleep: a post-marketing surveillance of 16944 cases. Int Clin Psychopharmacol 1998;13(4):157-167. 9727726ArticlePubMed
- 15. Roth T, Roehrs T, Vogel G. Zolpidem in the treatment of transient insomnia: a double-blind, randomized comparison with placebo. Sleep 1995;18(4):246-251. 7618022ArticlePubMed
- 16. Maarek L, Cramer P, Attali P, Coquelin JP, Morselli PL. The safety and efficacy of zolpidem in insomniac patients: a long-term open study in general practice. J Int Med Res 1992;20(2):162-170. 1521672ArticlePubMedPDF
- 17. Allain H, Monti J. General safety profile of zolpidem: safety in elderly, overdose and rebound effects. Eur Psychiatry 1997;12(Suppl 1):21-29. 19698571Article
- 18. Lee YJ. Overview of the therapeutic management of insomnia with zolpidem. CNS Drugs 2004;18(Suppl 1):17-23. 15291010ArticlePubMed
- 19. Vermeeren A. Residual effects of hypnotics: epidemiology and clinical implications. CNS Drugs 2004;18(5):297-328. 15089115ArticlePubMed
- 20. Mendelson WB. Sleepwalking associated with zolpidem. J Clin Psychopharmacol 1994;14(2):150. 8195460ArticlePubMed
- 21. Iruela LM. Zolpidem and sleepwalking. J Clin Psychopharmacol 1995;15(3):223. 7636001Article
- 22. Hoque R, Chesson AL Jr. Zolpidem-induced sleepwalking, sleep related eating disorder, and sleep-driving: fluorine-18-flourodeoxyglucose positron emission tomography analysis, and a literature review of other unexpected clinical effects of zolpidem. J Clin Sleep Med 2009;5(5):471-476. 19961034ArticlePubMedPMC
- 23. Yang W, Dollear M, Muthukrishnan SR. One rare side effect of zolpidem--sleepwalking: a case report. Arch Phys Med Rehabil 2005;86(6):1265-1266. 15954071ArticlePubMed
- 24. Buscemi N, Vandermeer B, Friesen C, Bialy L, Tubman M, Ospina M, et al. Manifestations and management of chronic insomnia in adults. Evid Rep Technol Assess (Summ) 2005;(125):1-10. 15989374
- 25. Zint K, Haefeli WE, Glynn RJ, Mogun H, Avorn J, Sturmer T. Impact of drug interactions, dosage, and duration of therapy on the risk of hip fracture associated with benzodiazepine use in older adults. Pharmacoepidemiol Drug Saf 2010;19(12):1248-1255. 20931664ArticlePubMedPMC
- 26. Glass J, Lanctot KL, Herrmann N, Sproule BA, Busto UE. Sedative hypnotics in older people with insomnia: meta-analysis of risks and benefits. BMJ 2005;331(7526):1169. 16284208ArticlePubMedPMC
- 27. Wang PS, Bohn RL, Glynn RJ, Mogun H, Avorn J. Zolpidem use and hip fractures in older people. J Am Geriatr Soc 2001;49(12):1685-1690. 11844004ArticlePubMed
- 28. Antai-Otong D. The art of prescribing. Risks and benefits of non-benzodiazepine receptor agonists in the treatment of acute primary insomnia in older adults. Perspect Psychiatr Care 2006;42(3):196-200. 16916422ArticlePubMed
- 29. Ki M. Theory and practice of case-crossover study design. Korean J Epidemiol 2008;30(1):1-11. (Korean)ArticlePDF
- 30. Maclure M. The case-crossover design: a method for studying transient effects on the risk of acute events. Am J Epidemiol 1991;133(2):144-153. 1985444ArticlePubMed
- 31. Maclure M. 'Why me?' versus 'why now?' Differences between operational hypotheses in case-control versus case-crossover studies. Pharmacoepidemiol Drug Saf 2007;16(8):850-853. 17636552ArticlePubMed
- 32. McMahon AD, Evans JM, McGilchrist MM, McDevitt DG, Mac-Donald TM. Drug exposure risk windows and unexposed comparator groups for cohort studies in pharmacoepidemiology. Pharmacoepidemiol Drug Saf 1998;7(4):275-280. 15073990ArticlePubMed
- 33. Tinetti ME. Clinical practice. Preventing falls in elderly persons. N Engl J Med 2003;348(1):42-49. 12510042ArticlePubMed
- 34. Frost M, Abrahamsen B, Masud T, Brixen K. Risk factors for fracture in elderly men: a population-based prospective study. Osteoporos Int 2012;23(2):521-531. 21409435ArticlePubMed
- 35. Hong GR, Cho SH, Tak Y. Falls among Koreans 45 years of age and older: incidence and risk factors. J Adv Nurs 2010;66(9):2014-2024. 20626472ArticlePubMed
- 36. Chang CM, Chen MJ, Tsai CY, Ho LH, Hsieh HL, Chau YL, et al. Medical conditions and medications as risk factors of falls in the inpatient older people: a case-control study. Int J Geriatr Psychiatry 2011;26(6):602-607. 21480377ArticlePubMed
- 37. Vitry AI, Hoile AP, Gilbert AL, Esterman A, Luszcz MA. The risk of falls and fractures associated with persistent use of psychotropic medications in elderly people. Arch Gerontol Geriatr 2010;50(3):e1-e4. 19423174ArticlePubMed
- 38. Richey SM, Krystal AD. Pharmacological advances in the treatment of insomnia. Curr Pharm Des 2011;17(15):1471-1475. 21476952ArticlePubMed
- 39. Wagner J, Wagner ML, Hening WA. Beyond benzodiazepines: alternative pharmacologic agents for the treatment of insomnia. Ann Pharmacother 1998;32(6):680-691. 9640488ArticlePubMed
- 40. Ravera S, van Rein N, de Gier JJ, de Jong-van den Berg LT. Road traffic accidents and psychotropic medication use in The Netherlands: a case-control study. Br J Clin Pharmacol 2011;72(3):505-513. 21501214ArticlePubMedPMC
REFERENCES
Figure 1Process for selecting study subjects. HIRA, Health Insurance Review and Assessment Service; ICD-10, International Statistical Classification of Diseases and Related Health Problems, 10th revision; ER, Emergency room; OS, orthopedics.
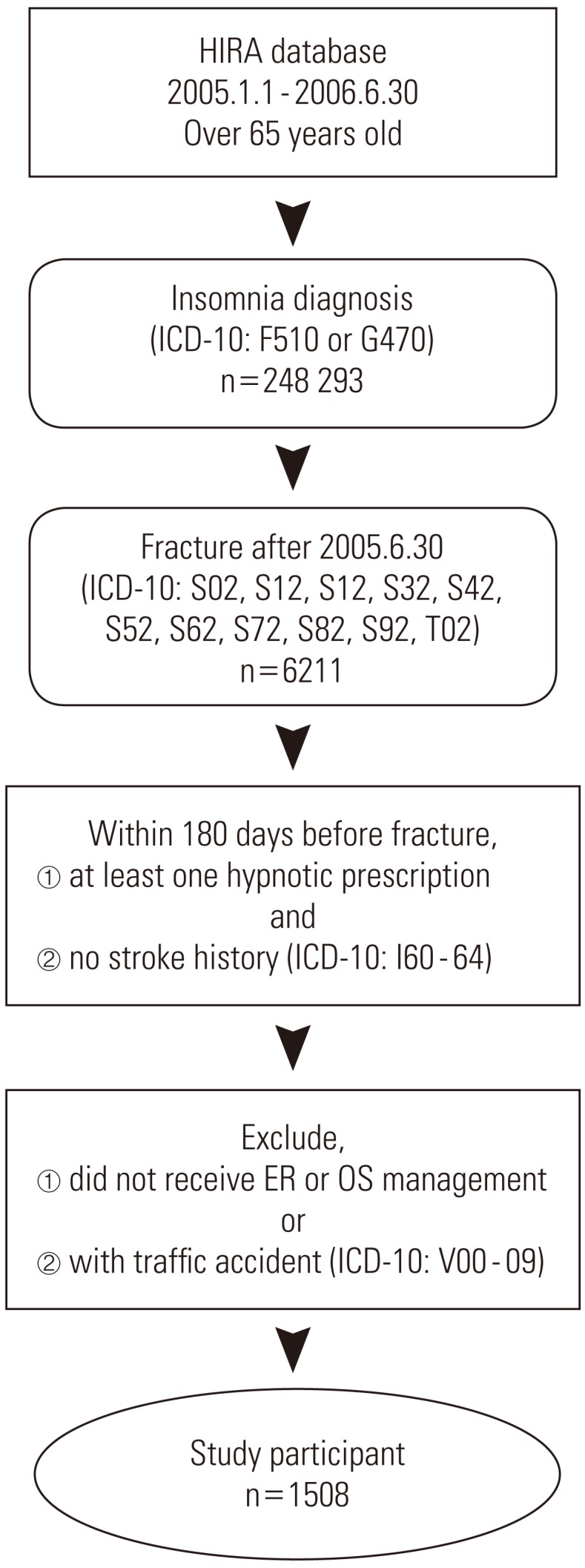

Figure 3Control of study period. 1Subjects should have more than one hypnotic prescription and no stroke history within this 180 days.


Table 1.Baseline characteristics of study patients (n=1508)
Table 2.Risk of fractures from hypnotic use in elderly insomnia patients stratified with a generic subgroup
| Hypnotics | No. of hazard period expo- sures (n = 1508) | No. of control period expo- sures (n = 6032) | Crude OR (95% CI) | Adjusted OR1 (95% CI) |
|---|---|---|---|---|
| Zolpidem | 236 | 722 | 1.84 (1.47, 2.30) | 1.72 (1.37, 2.16) |
| Benzodiazepin hypnotics | 364 | 1394 | 1.12 (0.93, 1.34) | 1.00 (0.83, 1.21) |
| Triazolam | 203 | 765 | 1.15 (0.91, 1.47) | 1.04 (0.82, 1.33) |
| Lorazepam | 146 | 550 | 1.17 (0.87, 1.58) | 1.06 (0.79, 1.44) |
| Flurazepam | 23 | 101 | 0.85 (0.48, 1.54) | 0.73 (0.41, 1.31) |
| Flunitrazepam | 25 | 108 | 0.81 (0.40, 1.66) | 0.78 (0.38, 1.58) |
| Midazolam | 3 | 15 | 0.69 (0.15, 3.30) | 0.60 (0.12, 2.92) |
| Brotizolam | 7 | 27 | 1.07 (0.35, 3.31) | 0.97 (0.31, 2.97) |
Table 3.Risk of fractures from hypnotic use in elderly insomnia patients stratified by age group and gender
Figure & Data
References
Citations
Citations to this article as recorded by 

- Sedative-hypnotics and osteoporotic fractures: A systematic review of observational studies with over six million individuals
Chong Xu, Janice Ching Nam Leung, Jiaying Shi, Dawn Hei Lum, Francisco Tsz Tsun Lai
Sleep Medicine Reviews.2024; 73: 101866. CrossRef - Burden of narcolepsy in Japan: A health claims database study evaluating direct medical costs and comorbidities
Yuta Kamada, Aya Imanishi, Shih-Wei Chiu, Takuhiro Yamaguchi
Sleep Medicine.2024; 114: 119. CrossRef - Analysis of Drugs Prescribed to Elderly Patients in a Tertiary Health Care Center in Raipur, Central India: An Observational Study
Yogendra Keche, Nitin R Gaikwad, Preetam N Wasnik, Keshao Nagpure, Md Sabah Siddiqui, Apoorva Joshi, Suryaprakash Dhaneria, Gevesh Dewangan, Jhasaketan Meher, Pranita Das
Cureus.2024;[Epub] CrossRef - Comparative risk of fracture in community‐dwelling older adults initiating suvorexant versus Z‐drugs: Results from LIFE study
Motohiko Adomi, Megumi Maeda, Fumiko Murata, Haruhisa Fukuda
Journal of the American Geriatrics Society.2023; 71(1): 109. CrossRef - Analysis of the prescription trends of potentially inappropriate medications in Korean older outpatients by sex: A retrospective study using data from the health insurance review and assessment service
Jae-Yong Dong, Jin-Han Ju, Young-Mo Yang
Medicine.2023; 102(34): e34818. CrossRef - Effectiveness of suvorexant versus benzodiazepine receptor agonist sleep drugs in reducing the risk of hip fracture: Findings from a regional population-based cohort study
Ryozo Yoshioka, Seiichiro Yamamoto, Eiji Nakatani, Norio Yasui-Furukori
PLOS ONE.2023; 18(4): e0284726. CrossRef - The epidemiology of new persistent hypnotic/sedative use after surgical procedures: a retrospective cohort study
D. H. Magnusson, T. I. Albertsson, F. Jonsdottir, M. I. Sigurdsson
Anaesthesia.2023; 78(8): 995. CrossRef - Professionals' treatment goals for long-term benzodiazepine and Z-drugs management: a qualitative study
Pauline Van Ngoc, Melissa Ceuterick, Jean-Luc Belche, Beatrice Scholtes
BJGP Open.2023; : BJGPO.2023.0034. CrossRef - Zolpidem as a high risk factor for elderly suicide in South Korea
Eun Kim, Jae Hee Lee, Duk Hee Lee
Archives of Suicide Research.2022; 26(2): 831. CrossRef - Reducing Sedative-Hypnotics Among Hospitalized Patients: a Multi-centered Study
Christine Soong, Cheryl Ethier, Yuna Lee, Dalia Othman, Lisa Burry, Peter E. Wu, Karen A. Ng, John Matelski, Barbara Liu
Journal of General Internal Medicine.2022; 37(10): 2345. CrossRef - Very long-term outcome of cognitive behavioral therapy for insomnia: one- and ten-year follow-up of a randomized controlled trial
Susanna Jernelöv, Kerstin Blom, Nils Hentati Isacsson, Pontus Bjurner, Ann Rosén, Martin Kraepelien, Erik Forsell, Viktor Kaldo
Cognitive Behaviour Therapy.2022; 51(1): 72. CrossRef - Efficacy and safety of Z-substances in the management of insomnia in older adults: a systematic review for the development of recommendations to reduce potentially inappropriate prescribing
Vincenz Scharner, Lukas Hasieber, Andreas Sönnichsen, Eva Mann
BMC Geriatrics.2022;[Epub] CrossRef - Insomnia Diagnosis, Prescribed Hypnotic Medication Use, and Risk for Serious Fall Injuries in the Reasons for Geographic and Racial Differences in Stroke (REGARDS) Study
S Justin Thomas, Swati Sakhuja, Lisandro D Colantonio, Mei Li, Paul Muntner, Kristi Reynolds, C Barrett Bowling
Sleep.2022;[Epub] CrossRef - Potentially Inappropriate Medication Use in Patients with Dementia
Kyungwon Yoon, Jung-Tae Kim, Won-Gun Kwack, Donghyun Kim, Kyung-Tae Lee, Seungwon Yang, Sangmin Lee, Yeo-Jin Choi, Eun-Kyoung Chung
International Journal of Environmental Research and Public Health.2022; 19(18): 11426. CrossRef - Z-drugs and falls in nursing home patients: data from the INCUR study
Sarah Damanti, Moreno Tresoldi, Philipe de Souto Barreto, Yves Rolland, Matteo Cesari
Aging Clinical and Experimental Research.2022; 34(12): 3145. CrossRef - Efficacy and Safety of Daridorexant in Older and Younger Adults with Insomnia Disorder: A Secondary Analysis of a Randomised Placebo-Controlled Trial
Ingo Fietze, Claudio L. A. Bassetti, David W. Mayleben, Scott Pain, Dalma Seboek Kinter, William V. McCall
Drugs & Aging.2022; 39(10): 795. CrossRef - Reacciones adversas medicamentosas de los hipnóticos más utilizados en España
A.J. Pardo-Cabello, V. Manzano-Gamero, J.D. Luna-del Castillo
Revista Clínica Española.2021; 221(2): 128. CrossRef - Adverse drug reactions among the most used hypnotic drugs in Spain
A.J. Pardo-Cabello, V. Manzano-Gamero, J.D. Luna-Del Castillo
Revista Clínica Española (English Edition).2021; 221(2): 128. CrossRef - Non-benzodiazepine hypnotic use for sleep disturbance in people aged over 55 years living with dementia: a series of cohort studies
Kathryn Richardson, George M Savva, Penelope J Boyd, Clare Aldus, Ian Maidment, Eduwin Pakpahan, Yoon K Loke, Antony Arthur, Nicholas Steel, Clive Ballard, Robert Howard, Chris Fox
Health Technology Assessment.2021; 25(1): 1. CrossRef - Sleep Disturbance, Sleep Disorders and Co-Morbidities in the Care of the Older Person
Christine E. Mc Carthy
Medical Sciences.2021; 9(2): 31. CrossRef - Effectiveness of App-Based Yoga of Immortals (YOI) Intervention for Insomnia in Asian Population during Pandemic Restrictions
Renuka Tunuguntla, Hari Siva Gurunadha Rao Tunuguntla, Himanshu Kathuria, Sadhna Verma
International Journal of Environmental Research and Public Health.2021; 18(11): 5706. CrossRef - Effects of anticholinergic and sedative medication use on fractures: A self‐controlled design study
Shahar Shmuel, Virginia Pate, Marc J. Pepin, Janine C. Bailey, Yvonne M. Golightly, Laura C. Hanson, Til Stürmer, Rebecca B. Naumann, Danijela Gnjidic, Jennifer L. Lund
Journal of the American Geriatrics Society.2021; 69(11): 3212. CrossRef - Effect of Multimorbidity on Fragility Fractures in Community-Dwelling Older Adults: Shimane CoHRE Study
Garu A, Shozo Yano, Abdullah Md Sheik, Aorigele Yu, Kenta Okuyama, Miwako Takeda, Kunie Kohno, Masayuki Yamasaki, Minoru Isomura, Toru Nabika, Atsushi Nagai
Journal of Clinical Medicine.2021; 10(15): 3225. CrossRef - Clinical consequences of abuse and misuse of hypnotics and analgesics in geriatric population
Paulina Trawka, Jakub Husejko, Kornelia Kędziora-Kornatowska
BÓL.2021; 22(2): 1. CrossRef - Exploring the Mechanisms Underlying Drug-Induced Fractures Using the Japanese Adverse Drug Event Reporting Database
Shinya Toriumi, Akinobu Kobayashi, Hitoshi Sueki, Munehiro Yamamoto, Yoshihiro Uesawa
Pharmaceuticals.2021; 14(12): 1299. CrossRef - Efficacy and safety of non-benzodiazepine and non-Z-drug hypnotic medication for insomnia in older people: a systematic literature review
Judith Sys, Simon Van Cleynenbreugel, Mieke Deschodt, Lorenz Van der Linden, Jos Tournoy
European Journal of Clinical Pharmacology.2020; 76(3): 363. CrossRef - Association between benzodiazepines use and risk of hip fracture in the elderly people: A meta-analysis of observational studies
Tahmina Nasrin Poly, Md. Mohaimenul Islam, Hsuan-Chia Yang, Yu-Chuan (Jack) Li
Joint Bone Spine.2020; 87(3): 241. CrossRef - Association entre prise de benzodiazépines et risque de fracture de la hanche chez la personne âgée : méta-analyse d’études observationnelles
Tahmina Nasrin Poly, Md. Mohaimenul Islam, Hsuan-Chia Yang, Yu-Chuan (Jack) Li
Revue du Rhumatisme.2020; 87(3): 210. CrossRef - Acute cognitive effects of the hypocretin receptor antagonist almorexant relative to zolpidem and placebo: a randomized clinical trial
Thomas C Neylan, Anne Richards, Thomas J Metzler, Leslie M Ruoff, Jonathan Varbel, Aoife O’Donovan, Melinda Sivasubramanian, Terri Motraghi, Jennifer Hlavin, Steven L Batki, Sabra S Inslicht, Kristin Samuelson, Stephen R Morairty, Thomas S Kilduff
Sleep.2020;[Epub] CrossRef - More than a quarter century of the most prescribed sleeping pill: Systematic review of zolpidem use by older adults
Flávio V. Machado, Luciana L. Louzada, Nathan E. Cross, Einstein F. Camargos, Thien Thanh Dang-Vu, Otávio T. Nóbrega
Experimental Gerontology.2020; 136: 110962. CrossRef - Association of Hypnotic Drug Use with Fall Incidents in Hospitalized Elderly Patients: A Case-Crossover Study
Haruki Torii, Motozumi Ando, Hideaki Tomita, Tomoko Kobaru, Mahoko Tanaka, Kazuhide Fujimoto, Rumiko Shimizu, Hiroaki Ikesue, Satoshi Okusada, Tohru Hashida, Noriaki Kume
Biological and Pharmaceutical Bulletin.2020; 43(6): 925. CrossRef - Treatment of Sleep Disturbance May Reduce the Risk of Future Probable Alzheimer’s Disease
Shanna L. Burke, Tianyan Hu, Christine E. Spadola, Aaron Burgess, Tan Li, Tamara Cadet
Journal of Aging and Health.2019; 31(2): 322. CrossRef - Twelve-year trend in the use of zolpidem and physicians’ non-compliance with recommended duration: a Korean national health insurance database study
Yunjeung Jang, Inmyung Song, In-Sun Oh, Ju-Young Shin
European Journal of Clinical Pharmacology.2019; 75(1): 109. CrossRef - Association between Elimination Half-life of Benzodiazepines and Falls in the Elderly: A Meta-analysis of Observational Studies
Chikako Masudo, Yukari Ogawa, Naomi Yamashita, Kiyoshi Mihara
YAKUGAKU ZASSHI.2019; 139(1): 113. CrossRef - Effectiveness and safety of fire-needle moxibustion on insomnia
Cuiling Liu, Zhiqiang Chen, Ting Li, Zhihua Yang, Qingsong Zhang, Jianping Yin, Peng Zhou, Wei Fu, BaiShu Chen
Medicine.2019; 98(7): e14509. CrossRef - Treatment of sleep disturbance in older adults
Amy C. Reynolds, Robert J. Adams
Journal of Pharmacy Practice and Research.2019; 49(3): 296. CrossRef - An Implementation Guide to Promote Sleep and Reduce Sedative-Hypnotic Initiation for Noncritically Ill Inpatients
Christine Soong, Lisa Burry, Hyung J. Cho, Evelyn Gathecha, Flora Kisuule, Cara Tannenbaum, Abi Vijenthira, Timothy Morgenthaler
JAMA Internal Medicine.2019; 179(7): 965. CrossRef - Evaluation of Dissolution Profile between Original and Generic Products of Zolpidem Tartrate by Microdialysis-HPLC
Kazunori Inaba, Toshiharu Oie, Hiroko Otake, Takeshi Kotake, Noriaki Nagai
Chemical and Pharmaceutical Bulletin.2019; 67(2): 120. CrossRef - Insomnia, Benzodiazepine Use, and Falls among Residents in Long-term Care Facilities
Jiang, Xia, Wang, Zhou, Jiang, Diwan, Xu
International Journal of Environmental Research and Public Health.2019; 16(23): 4623. CrossRef - Association Between Sleep Medications and Falls and Fall-related Worries in Community-Dwelling Older Adults in the United States
Kathy Nguyen, Jonathan Watanabe
Journal of Contemporary Pharmacy Practice.2019; 66(3): 23. CrossRef - Z-drugs and risk for falls and fractures in older adults—a systematic review and meta-analysis
Nir Treves, Amichai Perlman, Lital Kolenberg Geron, Angham Asaly, Ilan Matok
Age and Ageing.2018; 47(2): 201. CrossRef - Age, Sex, and Dose Effects of Nonbenzodiazepine Hypnotics on Hip Fracture in Nursing Home Residents
David D. Dore, Andrew R. Zullo, Vincent Mor, Yoojin Lee, Sarah D. Berry
Journal of the American Medical Directors Association.2018; 19(4): 328. CrossRef - Cognitive Enhancers Associated with Decreased Risk of Injury in Patients with Dementia: A Nationwide Cohort Study in Taiwan
Pei-Chun Chao, Wu-Chien Chien, Chi-Hsiang Chung, Ching-Wen Chu, Chin-Bin Yeh, San-Yuan Huang, Ru-Band Lu, Hsin-An Chang, Yu-Chen Kao, Hui-Wen Yeh, Wei-Shan Chiang, Yu-Ching Chou, Nian-Sheng Tzeng
Journal of Investigative Medicine.2018; 66(3): 684. CrossRef - Insomnia in Elderly Patients: Recommendations for Pharmacological Management
Vivien C. Abad, Christian Guilleminault
Drugs & Aging.2018; 35(9): 791. CrossRef - Mild cognitive impairment: associations with sleep disturbance, apolipoprotein e4, and sleep medications
Shanna L. Burke, Tianyan Hu, Christine E. Spadola, Tan Li, Mitra Naseh, Aaron Burgess, Tamara Cadet
Sleep Medicine.2018; 52: 168. CrossRef - An effectiveness comparison of acupuncture treatments for insomnia disorder
Jing Chen, Liming Lu, Nenggui Xu, Jun Chen, Yupeng Fan, Fen Feng, Xiaolan Qin, Yu Kui
Medicine.2018; 97(35): e12060. CrossRef - Zolpidem-Induced Acute Altered Level of Consciousness: A Report of Two Cases
Abdolhamid Parsa, Seyyed Ali Tabaeian, Sadra Einizadeh, Mohammad Babaeian
Iranian Journal of Psychiatry and Behavioral Sciences.2018;[Epub] CrossRef - Hypnotic drug risks of mortality, infection, depression, and cancer: but lack of benefit
Daniel F. Kripke
F1000Research.2018; 5: 918. CrossRef - Effects of Ramelteon and Other Sleep-Promoting Drugs on Serum Low-Density Lipoprotein and Non-high-density Lipoprotein Cholesterol: A Retrospective Comparative Pilot Study
Haruki Torii, Rumiko Shimizu, Yuriko Tanizaki, Yurina Omiya, Miwa Yamamoto, Sayaka Kamiike, Daisuke Yasuda, Yoshinori Hiraoka, Tohru Hashida, Noriaki Kume
Biological and Pharmaceutical Bulletin.2018; 41(12): 1778. CrossRef - Zopiclone Use and Risk of Fractures in Older People: Population-Based Study
Prasad S. Nishtala, Te-yuan Chyou
Journal of the American Medical Directors Association.2017; 18(4): 368.e1. CrossRef - Hypnotic drug risks of mortality, infection, depression, and cancer: but lack of benefit
Daniel F. Kripke
F1000Research.2017; 5: 918. CrossRef - Benzodiazepines, Z-drugs and the risk of hip fracture: A systematic review and meta-analysis
Karen Donnelly, Robert Bracchi, Jonathan Hewitt, Philip A. Routledge, Ben Carter, Tuan Van Nguyen
PLOS ONE.2017; 12(4): e0174730. CrossRef - Benzodiazepines and Z-Drugs: An Updated Review of Major Adverse Outcomes Reported on in Epidemiologic Research
Jaden Brandt, Christine Leong
Drugs in R&D.2017; 17(4): 493. CrossRef - Sleep in the Elderly
Steven H. Feinsilver, Adam B. Hernandez
Clinics in Geriatric Medicine.2017; 33(4): 579. CrossRef - A Greater Extent of Insomnia Symptoms and Physician-Recommended Sleep Medication Use Predict Fall Risk in Community-Dwelling Older Adults
Tuo-Yu Chen, Soomi Lee, Orfeu M Buxton
Sleep.2017;[Epub] CrossRef - High Prevalence of Inappropriate Benzodiazepine and Sedative Hypnotic Prescriptions among Hospitalized Older Adults
Elisabeth Anna Pek, Andrew Remfry, Ciara Pendrith, Chris Fan‐Lun, R. Sacha Bhatia, Christine Soong
Journal of Hospital Medicine.2017; 12(5): 310. CrossRef - Zolpidem use and risk of fractures: a systematic review and meta-analysis
S. M. Park, J. Ryu, D. R. Lee, D. Shin, J. M. Yun, J. Lee
Osteoporosis International.2016; 27(10): 2935. CrossRef - Melatonin, hypnotics and their association with fracture: a matched cohort study
Martin Frisher, Nicholas Gibbons, James Bashford, Steve Chapman, Scott Weich
Age and Ageing.2016; 45(6): 801. CrossRef - Characteristics and Trends in Hypnotics Consumption in the Largest Health Care System in Israel
O. Marom, G. Rennert, N. Stein, K. Landsman, G. Pillar
Sleep Disorders.2016; 2016: 1. CrossRef - Screening Tool for Older Persons’ Appropriate Prescriptions for Japanese: Report of the Japan Geriatrics Society Working Group on “Guidelines for medical treatment and its safety in the elderly”
Taro Kojima, Katsuyoshi Mizukami, Naoki Tomita, Hiroyuki Arai, Takashi Ohrui, Masato Eto, Yasushi Takeya, Yoshitaka Isaka, Hiromi Rakugi, Noriko Sudo, Hidenori Arai, Hiroaki Aoki, Shigeo Horie, Shinya Ishii, Koh Iwasaki, Shin Takayama, Yusuke Suzuki, Tosh
Geriatrics & Gerontology International.2016; 16(9): 983. CrossRef - Review of Safety and Efficacy of Sleep Medicines in Older Adults
Jennifer L. Schroeck, James Ford, Erin L. Conway, Kari E. Kurtzhalts, Megan E. Gee, Krista A. Vollmer, Kari A. Mergenhagen
Clinical Therapeutics.2016; 38(11): 2340. CrossRef - Nonbenzodiazepine Sedative Hypnotics and Risk of Fall-Related Injury
Sarah E. Tom, Emerson M. Wickwire, Yujin Park, Jennifer S. Albrecht
Sleep.2016; 39(5): 1009. CrossRef - Hypnotic drug risks of mortality, infection, depression, and cancer: but lack of benefit
Daniel F. Kripke
F1000Research.2016; 5: 918. CrossRef - Dependence, misuse, and beliefs regarding use of hypnotics by elderly psychiatric patients taking zolpidem, estazolam, or flunitrazepam
Cheng-Fang Yen, Chih-Hung Ko, Yu-Ping Chang, Cheng-Ying Yu, Mei-Feng Huang, Yi-Chun Yeh, Jin-Jia Lin, Cheng-Sheng Chen
Asia-Pacific Psychiatry.2015; 7(3): 298. CrossRef - An Increased Risk of Reversible Dementia May Occur After Zolpidem Derivative Use in the Elderly Population
Hsin-I Shih, Che-Chen Lin, Yi-Fang Tu, Chia-Ming Chang, Hsiang-Chin Hsu, Chih-Hsien Chi, Chia-Hung Kao
Medicine.2015; 94(17): e809. CrossRef - The Use of Hypnotics to Treat Sleep Problems in the Elderly
Catherine McCall, John W. Winkelman
Psychiatric Annals.2015;[Epub] CrossRef - Risk of Type 2 Diabetes in Patients With Nonapnea Sleep Disorders in Using Different Types of Hypnotics
Chia-Ling Lin, Mei-Chang Yeh, Tomor Harnod, Cheng-Li Lin, Chia-Hung Kao
Medicine.2015; 94(38): e1621. CrossRef - Care Needs and Clinical Outcomes of Older People with Dementia: A Population-Based Propensity Score-Matched Cohort Study
Fei-Yuan Hsiao, Li-Ning Peng, Yu-Wen Wen, Chih-Kuang Liang, Pei-Ning Wang, Liang-Kung Chen, Alessandra Marengoni
PLOS ONE.2015; 10(5): e0124973. CrossRef - Is Zolpidem Associated with Increased Risk of Fractures in the Elderly with Sleep Disorders? A Nationwide Case Cross-Over Study in Taiwan
Yih-Jing Tang, Shinn-Ying Ho, Fang-Ying Chu, Hung-An Chen, Yun-Ju Yin, Hua-Chin Lee, William Cheng-Chung Chu, Hui-Wen Yeh, Wei-Shan Chiang, Chia-Lun Yeh, Hui-Ling Huang, Nian-Sheng Tzeng, Uwe Rudolph
PLOS ONE.2015; 10(12): e0146030. CrossRef - Evaluation of risk factors for fractures in postmenopausal women with osteoporosis
Viktória Ferencz, Csaba Horváth, Sándor Huszár, Katalin Bors
Orvosi Hetilap.2015; 156(4): 146. CrossRef - Association between use of benzodiazepines and risk of fractures: a meta-analysis
D. Xing, X. L. Ma, J. X. Ma, J. Wang, Y. Yang, Y. Chen
Osteoporosis International.2014; 25(1): 105. CrossRef - Non-benzodiazepine hypnotics and older adults: what are we learning about zolpidem?
Hedva B Levy
Expert Review of Clinical Pharmacology.2014; 7(1): 5. CrossRef - Long-Term Use of Zolpidem Increases the Risk of Major Injury: A Population-Based Cohort Study
Ming-May Lai, Cheng-Chieh Lin, Che-Chen Lin, Chiu-Shong Liu, Tsai-Chung Li, Chia-Hung Kao
Mayo Clinic Proceedings.2014; 89(5): 589. CrossRef - Risk of hip fracture among older people using anxiolytic and hypnotic drugs: a nationwide prospective cohort study
Marit Stordal Bakken, Anders Engeland, Lars B. Engesæter, Anette Hylen Ranhoff, Steinar Hunskaar, Sabine Ruths
European Journal of Clinical Pharmacology.2014; 70(7): 873. CrossRef - Treatment of Insomnia in Older Adults: Re-Evaluating the Benefits and Risks of Sedative Hypnotic Agents
Nicole J. Brandt, Jennifer M. Piechocki
Journal of Gerontological Nursing.2013; 39(4): 48. CrossRef - In the Zzz Zone: The Effects of Z-Drugs on Human Performance and Driving
Naren Gunja
Journal of Medical Toxicology.2013; 9(2): 163. CrossRef - Ten-year trend in prescriptions of z-hypnotics among the elderly: A nationwide, cross-sectional study in Taiwan
Fei-Yuan Hsiao, Pei-Hua Hsieh, Churn-Shiouh Gau
Journal of Clinical Gerontology and Geriatrics.2013; 4(2): 37. CrossRef - Zolpidem, Is it a Safe Drug for Insomnia Management?
Kyung Hee Park, Jae-Hyun Lee
Korean Journal of Medicine.2013; 84(6): 802. CrossRef
 KSPM
KSPM

 PubReader
PubReader ePub Link
ePub Link Cite
Cite