Comparison of Sexual Risky Factors of Men Who Have Sex With Men and Sex-buying Men as Groups Vulnerable to Sexually Transmitted Diseases
Article information
Abstract
Objectives
It is necessary to examine groups carrying out sexually risky behavior because the prevalence of sexually transmitted diseases (STDs) is high among them. In this study, the prevalence of STDs among homosexuals and sex-buying men in South Korea was investigated, along with their sexual risk factors.
Methods
Men who have sex with men (MSMs, n=108) were recruited in Seoul and Busan by applying the time location sampling method, while sex-buying men (n=118) were recruited from a john school in Gyeonggi province, the suburbs of Seoul. Dependent variables included past or present infection with syphilis, Chlamydia, gonorrhea, and human immunodeficiency virus. Independent variables included health behavior, social support, sexual behavior, and safe sex.
Results
It was found that when the MSMs were non-drunk while having sexual intercourse (odds ratio [OR], 0.132), they showed a higher STD infection rate when they had a higher number of anal sex partners (OR, 5.872), rarely used condoms (OR, 1.980), had lower self-efficacy (OR, 0.229), and were more anxious about becoming infected with an STD (OR, 3.723). However, the men who paid for sex showed high STD infections when they had more sex partners (OR, 2.286) and lower education levels (OR, 3.028).
Conclusions
STD infections among the two groups were high when they were engaged with many sex partners and not having protected sex. In other words, there was a gap in risky sex behavior within such groups, which was significantly related to the possibility of developing an STD. Therefore, the preventive intervention against STDs for these groups needs to be expanded to include management of sex behaviors.
INTRODUCTION
Sexually transmitted diseases (STDs) are major public health concerns that attract the attention of underdeveloped, developing, and developed countries alike. The World Health Organization (WHO) estimated that during the year 1999, 340 000 cases of sexual diseases like syphilis, gonorrhea, and Chlamydia have occurred, and that this number still has not been reduced in the 21st century [1,2]. It is not so easy to manage sexual diseases given that approaching the group estimated to have a high infection rate is not easy, nor is measuring the subjects' sexual behavior and infection rate. Patients with sexual diseases in the above mentioned groups are called an unseen community not only because the disease is hidden, but also because of the social stigma around sex [3,4]. Despite everything, preventative intervention for this is critical because it is evident that sexual risky behaviors such as having sexual relationships with multiple partners and not using condoms act as factors that increase the STD infection rate [5,6].
Risky sexual behaviors appear from a couple of groups that have differentiated characteristics from the normal population [7-9]. Of course, even in the case of general society, the sexual behaviors of urban residents, the unmarried, and adolescent groups are relatively dangerous [1]. The groups that have the tendency toward even riskier behaviors than those mentioned above are female sex workers (FSWs), men who have sex with men (MSMs), sex-buying men (SBMs), and immigrant workers [4]. Categorizing these groups as one requires much caution. FSWs, seen within the context of the sex industry, are in the position of submitting to sex, not enjoying sexual activities [10], and immigrant workers are driven to dangerous sexual activities due to the structural context in which they cannot meet sexual partners along with low social status and stress [11]. Therefore, considering the social aspect in which sexual behaviors and norms are developed, a sexual risky group with a high rate of sexually transmitted disease infection can be called a vulnerable group to sexual diseases rather than a socially hazardous group.
Until now, there has not been enough research on subjects vulnerable to sexual diseases. A sentinel surveillance system that has served to monitor for the outbreak of sexual diseases is available, but there has been a huge lack of sharing of research on sexual behaviors and information. This lack of understanding brings about social stigma and fear of human immunodeficiency virus (HIV)/acquired immunodeficiency syndrome (AIDS), leading subjects with sexual disease to further exclusion from the society and further vulnerability [4]. This research calculated the sexual disease prevalence rate among MSMs and SBMs in South Korea, and analyzed the sexual behavior risk factors that could affect transmission of sexual disease. The results of this study not only help to understand groups vulnerable to sexual disease, but also help to suggest implications for public health in terms of effective methods of prevention of sexual disease.
METHODS
I. Data Collection
The research data was collected through a study on the prevalence of STDs in SBM and MSM groups performed as a part of the "Prevalence of sexually transmitted diseases in high risk populations of Korea" study in the year 2008. First, MSMs were recruited from the Ivan Stop HIV/AIDS Project (iSHAP) in Seoul (n=49), iSHAP in the city of Busan (n=20), and from the Condom Café at a queer cultural event that took place in the neighborhood of Jongno-gu in Seoul (n=39). On the other hand, SBMs were recruited among students of John School of Gyeonggi province (n=118). John School is a kind of probation office where first offenders who have solicited prostitution are educated for 8 hours in order to prevent the recurrence of prostitution. Therefore, the two participant groups of this study are clearly differentiated from the general population. MSMs are defined as men who have actively participated in the gay community at present, and SBMs are defined as men who have experience of paying for sex.
The research was conducted by visiting the actual site 4 times from August to October of 2008. People involved in the data gathering visits included 1 doctor, 2 medical laboratory technologists, 2 people with a master's in public health and 1 Korea Federation for HIV/AIDS Prevention affiliated director, working as a team. While medical laboratory technologists were taking urine, oropharyngeal smears, and blood clinical specimens, a trained interviewer received answers from investigation participants on sexual behaviors. The biological clinical specimen was composed of treponema pallidum antibodies (TP-PA), urine polymeras chain reaction (PCR) for Chlamydia trachomatis and Neisseira gonorrhea, and an HIV test (4th generation enzyme immuno assays for HIV) although the SBMs were examined only for Chlamydia and gonorrhea. Pathological examination of the clinical specimens was performed at the Science Research Center in Seoul (SRC).
II. Ethical Issues
This research passed the institutional review board of Seoul National University Hospital in May 14th, 2008. The confirmation number was C-0801-047-232. In order to protect vulnerable research subjects, every actual site investigation was performed by receiving informed consent from the participants. During the investigation process, absolutely no information that could distinguish individual respondents was collected, and clinical specimens were classified using bar codes. Investigation results were made so that only the individual himself was able to confirm through the automatic response system of the SRC, and the secrecy of all information was fully assured.
III. Samples
The samples were collected through convenience sampling of data, but in the case of the MSMs, the time location sampling (TLS) was applied [3,12,13]. In this research, iSHAP and the Condom Café where MSMs gather most frequently were designated as the location of collecting samples. On the other hand, since SBMs are not an exclusive community has no social network or such inclinations, the John School, the only location where they are guaranteed to assemble, was used as the sampling frame.
IV. Measures
A sex behavior measurement instrument set the groundwork for behavioral surveillance surveys whose validity was verified through an internationally recognized process [14]. Items on safe sex measured the extent to which a person is confident about persuading his partner to wear a condom in case the partner does not wish to do so, in other words, self-efficacy [15] (standard group: low) as well as whether or not the partner used a condom (standard group: use). Besides this, the extent in which the respondent subjectively felt anxiety about sexually transmitted infection was measured using a Likert 3-point scale. However, because the confidence level related to persuading the partner to use condom for SBMs was inappropriate, by definition, thus the researchers did not measure it in their cases. In relation to the risky sexual behavior, the researchers asked how many sex partners the subjects had had sex with during the past one year. In order to do this, considering the characteristics of the partners, the researchers limited the partners of MSMs to anal intercourse partners only. In addition, were the MSMs were asked questions that involved requiring the subjects to answer whether or not they had sexual experience with female partners (standard group: no experience). In terms of social support, the subjects were asked to give the number of close people they keep in touch with in terms of an interval scale. In relation to health behavior, questions on drunk-driving and smoking, originating from the WHO, made into a 5-point scale were used. For smoking, the researchers asked whether or not the subjects smoked (standard group: yes to smoking), and for drunk sex, the subjects were asked if the participants were in a drunk state while they were having sex (standard group: yes to drunk sex experience).
The dependent variable was defined as whether or not the subjects had STDs, and it included all subjects diagnosed as having been infected with STDs at the present and in the past. The method used to grasp the number of subjects diagnosed with STDs at present was through the clinical specimen, and the number of subjects infected with STDs in the past was grasped through surveys performed at the same time. If a subject had been diagnosed with an STD at least once, then he was coded as 1, and a subject had never been diagnosed with an STD then he was coded as 0. Then the researchers examined the risk factors for STD infection through logistic regression analysis, which has the previously-mentioned variable as a dependent variable. The exposure period, sampling region (in the case of the MSMs), and education level (in the case of the SBMs) were used as confounding variables. The exposure period was defined by subtracting the first sexual experience age from the current age. The three locations where the MSMs were collected were processed as a dummy variable. In addition, the education level was added due to the fact that it is a predictive factor for people who have concurrent sexual partnership among the general population (standard group: college graduate or above).
V. Statistical Analysis
Analysis processes are as follows: 1) Descriptive statistics on general characteristics of the sample were calculated. 2) The prevalence rate of the major STDs of the investigation subjects was calculated. 3) Using binomial logistic regression analysis, the researchers examined the STD infection-related sex behavior risk factors of MSMs and SBMs as odds ratios and compared both. However, the number of sex partners was not normally distributed in either group, and thus it was put into the model after it was transformed to a log value.
RESULTS
I. General Characteristics of Samples
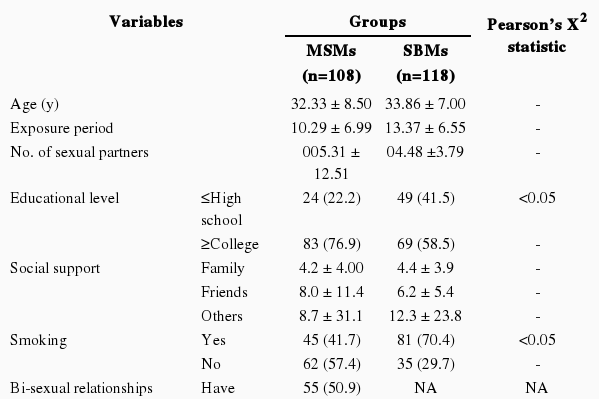
Age, on average, was almost the same in the MSMs (32.33±8.50) and SBMs (33.86±7.00) (Table 1). Regarding educational background, 83 (76.9%) of the MSMs were college graduates or above, and this value was a higher proportion than the 69 (58.5%) college graduates among the SBMs (p < 0.05). The average number of people among family members and friends the subjects kept in touch with among the MSMs was 4.2 to 8.7 and in the sex-buying group was 4.4 to 12.3, and those two numerical values were at similar levels to each other. In the case of smoking, 62 MSMs were nonsmokers (57.4%), but 81 SBMs (70.4%) were currently smokers (p<0.05). Among MSMs, 55 (50.9%) had had bisexual experience. The number of MSMs who had had a sexual relationship before the age of 19 was 35 (33.0%) which was higher than the 31 among the SBMs (26.5%). However, the exposure period calculated after having applied the above-mentioned numerical values for SBMs was 13.37 years, on average, which was longer than that of the MSMs at an average of 10.29 years. The MSMs had a greater number of partners in the past year, 5.31 on average, and this was higher than the 4.48 of the SBMs. However, the sex partners of the MSMs were limited to anal sex intercourse only, while the total number of sex partners was 9.12 (±16.62), on average. Regarding usage of condoms, the response 'almost use' for MSMs was 83 (83.0%), but only 57 among the SBMs (48.7%), which was very low (p < 0.05). On the subject of being drunk while having sexual intercourse, 51 MSMs (47.2%) said no, but 91 SBMs (77.3%) said yes (p < 0.05). Out of the total number of MSMs, 76 (73.8%) responded that their self-efficacy for using a condom was high. However, 31 SBMs (26.2%) expressed anxiety about getting infected by STDs, as did 42 MSMs (38.9%). The subjective health condition of both groups was satisfactory, but in the case of the MSMs, there was a higher response of "not healthy" (7.4%). In general, the MSMs had a higher ratio of practicing safe sex than the SBMs, although the frequency of sexual relationships and riskiness was higher.
II. Sexually Transmitted Disease Prevalence Rate
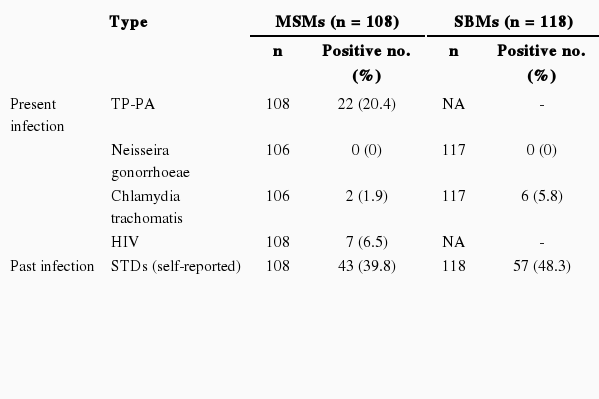
The prevalence rate, which was calculated by dividing the number of those testing positive for sexually transmitted diseases by the number of investigation subjects, is shown in Table 2. In the case of present infection, out of all the MSMs, the prevalence rate of syphilis was 20.4% (95% confidence interval [CI], 12.8 to 28.0), the prevalence rate of Chlamydia was 1.9% (95% CI, 0.7 to 4.5), and the prevalence rate of HIV was 6.5% (95% CI, 1.9 to 11.1). For gonorrhea, both PCR and an oropharyngeal cultivation test were performed, but all of the clinical specimens were negative. The SBMs were also all negative for gonorrhea and the prevalence rate of Chlamydia was 5.8% (95% CI, 3.7 to 7.5). On the other hand, out of all the MSMs, there were 43 who had previous STD infection experience (39.8%) and 65 answered "never" (60.2%). In the case of SBMs, the results were 57 (48.3%) and 61 (51.7%), respectively.
III. Comparison of a Sexually Risky Behavior Model Between MSMs and SBMs
The following are the results of a logistic regression analysis of the sex behavior factors related to the STD infection of the two vulnerable groups (Table 3). For the MSMs, the greater the number of sexual partners (odds ratio [OR], 5.87; 95% CI, 1.97 to 17.48), the more likely they were not to use condoms (OR, 1.98; 95% CI, 1.54 to 2.00), the more anxious they were about STD infection (OR, 3.72; 95% CI, 1.47 to 9.44), the longer the exposure period (OR, 1.22; 95% CI, 1.04 to 1.42), and the higher the possibility of risk of STD infection. On the other hand, the higher the self-efficacy (OR, 0.23; 95% CI, 0.06 to 0.82), the lower the possibility of STD infection risk.
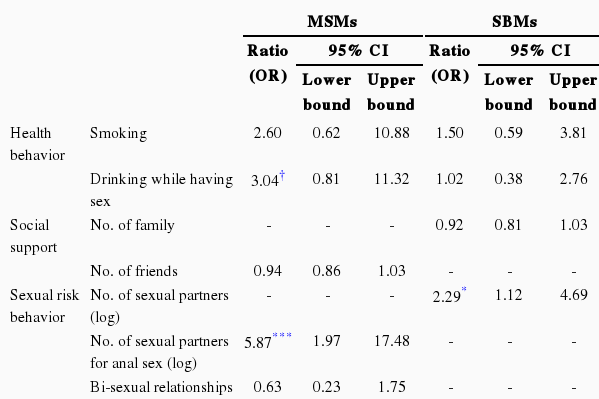
Sexual risk factors of present and past infection with sexually transmitted disease among MSMs and SBMs
In the case of SBMs, the more the number of sexual partners (OR, 2.29; 95% CI, 1.12 to 4.69) and the less formal education (OR, 3.03; 95% CI, 1.22 to 7.50), the higher the risk of STD infection. On the other hand, although marginally significant, the less they used condoms (OR, 1.38; 95% CI, 0.95 to 2.02) and the longer the exposure period (OR, 1.07; 95% CI, 0.99 to 1.16), the higher the possibility of STD infection risk.
DISCUSSION
This research was performed based on an investigation of the STD prevalence rate and sex behavior of both MSMs and SBMs of Korea. There have been previous local studies aimed at calculating these prevalence rates, but there was a huge scarcity of understanding of sex behaviors. There have been reports on sexually risky behavior in the general population [9], but since MSMs and SBMs are difficult to approach, research on actual sex behavior has not been conducted. We now discuss factors related to sampling investigation subjects as well as the STD prevalence rate of sexually-vulnerable male groups, as detailed below.
I. Participant Sampling
The investigation subjects of this research are hidden populations for whom statistical probability sampling is difficult. For this kind of group, snowball sampling (with random characteristics) has been used, and the recently developed respondent-driven sampling has not been applied or been established in Korea due to the difficulty in actual site application despite its advantage of being able to collect representative samples. Therefore, such a sample is basically a convenience sample. However, TLS method was used as the MSM sampling method. TLS is a sampling method suitable for subjects, like MSMs, who are approachable at certain locations. This method has been used often in instances where the location of the subjects had to be used as sample framework in order to approach subjects like FSWs who are assembled only in at limited location [12,13]. Due to the fact that TLS is a method which sets the basis on the tendency of unseen communities [3], it is an effective tool for the sampling of groups, like MSMs, who are minorities who assemble in designated locations. Up to now, in Korea, the TLS method, which was used in this study, has been a practical approach that allows researchers to sample groups vulnerable to STDs.
II. Sexually Transmitted Disease Prevalence Rate and Sex Behavior
Examining the STD prevalence rate, the current TP-PA cultivation rate of MSMs in our study was rather high at 20.4%. In comparing with other Asian countries, MSMs' syphilis prevalence rate in the Jiangsu region of China was 6.9% [16], which is a significantly higher numerical value than Bangladeshi MSMs' prevalence rate of 12.0% [17]. On the other hand, in the case of gonorrhea, neither MSMs nor SBMs had any positive clinical specimen results in our study. There have been reports of a 2.7% prevalence rate of STDs among MSMs in China [16], 2.8% in Nepali SBMs [18], and a 0.4% of prevalence rate in metropolitan area male university students in Korea [19].
In the present study, the prevalence rate of Chlamydia was 1.9% among MSMs, and 5.1% among SBMs. These figures are a low compared to the fact that the prevalence rate among MSMs in China was 8.0%. However, previous research in Korea on groups who had possibly paid for sex showed a figure around 5% for SBMs [19,20]; the results of our study were very similar. In addition, it should be noted that this figure was much lower than that of the FSWs (20.0%) who become the partners of SBMs [21].
It was shown that the HIV infection rate of MSMs was 6.5% in this study. Considering that the research done on MSMs in Bangladesh and China showed almost no incidence of HIV positive subjects [16,17], this figure can be said to be high. However, it is difficult to come up with a general conclusion about the STD prevalence rate, because the participants of this research were recruited through a sort of convenience sampling; nevertheless, we can say that the Korean MSMs come much closer to being part of an at-risk group than Chinese FSWs [22]. Therefore, this research aimed to pay close attention to the subjects who have experienced STD infection to see why and how they were infected based on differences in their sex behaviors. STDs are spread through sexual relationships, so there is a high possibility that differences among sex behavior could increase infection risk.
According to the sex behavior risk model of MSMs and SBMs deducted in this study, there exist similarities and differences between the two groups. First, the similarity of the two groups is that the decisive factor related to STD infection in both groups is the number of sexual partners. In the case of MSMs, if there were many anal sex partners, the infection risk grew to be 5.87 times than a baseline, and the risk was 2.29 times higher than normal individuals in the case of SBMs. Except for that factor, among the SBMs, no particular factor was discovered. However, with a low formal education level, the probability of STD infection grew to be 3.03 times higher. Interestingly enough, the education level in previous studies was not a significant factor determining the number of sex partners [9]. Thus, from the general population, more investigation is needed to find out what kind of males become SBMs, and the STD infection route of males whose socioeconomic status is low should also be explored.
Unlike the SBMs, the MSMs diagnosed with STDs showed various sex behavior characteristics. First, these men had an infection risk 1.22 times higher when the exposure period was long, and an infection risk 3.72 times higher for those more anxious about STD infection. Given that STD infection anxiety is a negative emotion [14] that arises in cases in which the subject did something that could increase STD infection risk, it could also be seen as a proxy indicator of how much risky sexual behavior contributes to the increase in the infection rate. Whether or not one reported performing safe sex behavior was also an important factor. If no condom was used, the probability of getting infected with an STD increased by 1.98 times. On the other hand, if the self-efficacy for the use of condoms was high, the probability of infection decreased by 0.23 times. However, whether or not the subjects had sex with the opposite sex, not only the same sex, was not a predictive factor for STD infection. Furthermore, differences among regions where the samples were collected did not exist.
When all of the findings are taken together and, it could be said that the MSMs in Korea had more sex partners and a much higher ratio of anal sex intercourse than that found in previous studies [9] that observed characteristics of a concurrent high-risk sex group. From the finding that there is a lower STD infection risk among two-sex-partners, it could be seen that STD infection occurs at a higher rate within the MSM community. Rather than the act of homosexuality of MSMs, from the fact that even the general population shows similar results when they have many sex partners [9], it is estimated that the act of anal sex intercourse itself increases the probability of STD infection. The MSMs in this study used condoms more frequently, had sexual intercourse less often while drunk, and practiced safer sex than SBMs. However, because the MSMs had a higher ratio of risky sexual behavior compared to people in the general population with many sex partners, their infection rate was still higher. At the same time, according to the sexual behavior comparison model, the MSMs and SBMs in Korea had quite different characteristics in terms of STD preventative practice and sexual behavior.
This study has limited generalizability because the sample size was small. Even when the researchers applied TLS, the sample was composed of MSMs who were known to show more risky sex behavior than has been shown previously. For example, according to other studies, it is known that 22% to 28% of MSMs in Korea do not perform anal intercourse [4]. However, in this study, 15.4% of MSMs responded that they do not perform anal intercourse when they meet someone for the first time, and 10.7% of MSMs said they do not perform anal intercourse when they have fixed partners. In other words, it seemed that the core group who performed more anal intercourse was recruited as into the sample. The high HIV positive rate also reflects this fact. Next, because we pooled the regression variables for the subjects who experienced STD infection in terms of the present and past, whether or not there could be differences in sex conduct after STD infection could not be reflected in the model.
This investigation explored the risk factors of sexual behavior related to STD infection and the STD prevalence rate of SBMs and MSMs. It was found that these samples both showed a higher STD prevalence rate than the general population. Differences in risky sexual behavior existed even among individuals within the two STD-vulnerable groups, and in cases where the risk level was higher, so was the probability of STD infection. Therefore, it is necessary to identify these vulnerable groups for targeting effective STD prevention programs. Rather than implementing such a program by relying on the prevalence rate only, we need to address their sexual behavior in terms of medical sociology. This will not only reduce their social stigma but it will also increase the conformity level and the effect of the program.
ACKNOWLEDGEMENTS
This work was supported by the National Research Foundation of Korea Grant funded by the Korean Government (NRF-2007-E00049-00).
Notes
The authors have no conflicts of interest with the material presented in this paper.
This article is available at http://jpmph.org/.