Arterial Stiffness is Associated With Diabetic Retinopathy in Korean Type 2 Diabetic Patients
Article information
Abstract
Objectives
We evaluated the association between common carotid artery intima-media thickness (CCA-IMT), brachial-ankle pulse wave velocity (baPWV), carotid plaque, and peripheral arterial disease (PAD) as indicators of macroangiopathy and diabetic retinopathy as an indicator of microangiopathy in type 2 diabetic patients.
Methods
We analyzed 605 type 2 diabetic patients registered at a public health center in Korea. Following overnight fasting, venous blood and urine samples were collected and analyzed. The CCA-IMT, levels of carotid plaque, baPWV, and ankle-brachial index (ABI) of the subjects were assessed. We used non-mydriatic fundus photography to diagnose diabetic retinopathy. Multiple logistic regression analyses were used to evaluate the association between macroangiopathy and diabetic retinopathy. CCA-IMT and baPWV were divided into tertiles: CCA-IMT, 0.39 to 0.65 mm, 0.66 to 0.78 mm, and 0.79 to 1.30 mm; baPWV, 9.9 to 15.8 m/s, 15.9 to 18.9 m/s, and 19.0 to 38.0 m/s.
Results
The association between baPWV and diabetic retinopathy remained significant after adjustment, with an increasing odds ratio (OR) in the second tertile (OR, 2.41; 95% confidence interval [CI], 1.27 to 4.55) and the third tertile (OR, 4.63; 95% CI, 2.33 to 9.21). No significant differences were observed in carotid plaque, PAD, and each tertile of CCA-IMT.
Conclusions
BaPWV was associated with diabetic retinopathy, while CCA-IMT, carotid plaque, and PAD were not. This study suggests that the association between macroangiopathy and microangiopathy may be attributable to functional processes rather than structural processes within the vascular system.
INTRODUCTION
Recent epidemiologic studies have shown a significant rise in the prevalence of type 2 diabetes worldwide, resulting in increased burdens on individuals and health care systems [1,2]. This disease creates huge social, economic, and health problems, caused mainly by diabetic macro- and microvascular complications [2,3]. The frequent coexistence of diabetic macro- and microangiopathy was described decades ago [4-6].
For macroangiopathy, several measures of subclinical atherosclerosis have been developed as surrogate measures of cardiovascular disease (CVD) [7-11]. Atherosclerosis consists of two distinct processes: a structural process (atherosis) and a functional process (sclerosis) [12]. Common carotid artery intima-media thickness (CCA-IMT), ankle-brachial index (ABI), and carotid plaque presence are considered parameters of structural changes in atherosclerosis whereas brachial-ankle pulse wave velocity (baPWV) is a convenient measure of functional changes in atherosclerosis [13]. Similar to subclinical atherosclerosis, diabetic microangiopathy is associated with CVD risk in type 2 diabetic patients [14-16].
Such evidence is supported by a "common mechanism" for the development of macro- and microangiopathy in type 2 diabetes [17-20]. Several studies have evaluated the association between macroangiopathy and diabetic retinopathy [20-24]. However, these associations have not been adequately explored, particularly in the Korean population.
In this study, we evaluated the association between CCA-IMT, baPWV, carotid plaque, and peripheral arterial disease (PAD) as indicators of macroangiopathy, and diabetic retinopathy as an indicator of microangiopathy in type 2 diabetic patients.
METHODS
I. Subjects
The study population consisted of 1275 type 2 diabetic patients registered at a public health center of Seo-gu, Gwangju, and Gokseong-gun, Jeollanamdo, Korea. The subjects were participants in the surveys of the Seo-gu study (conducted between November 2008 and December 2008), and the Gokseong-gun study (conducted between June 2009 and November 2009).
In total, 1275 eligible subjects (Seo-gu: 681, Gokseong-gun: 594) were invited by telephone to participate. Of these, 709 (Seo-gu: 327, Gokseong-gun: 382) underwent clinical examinations following interviews. The response rate for the study was 55% (Seo-gu: 48.0%, Gokseong-gun: 64.3%). Ten patients who did not provide blood samples and twenty-six who did not provide urine samples were excluded from the study. Sixty-eight patients had no HbA1c, CCA-IMT, carotid plaque, baPWV, history of diabetic duration, or fundoscopic outcome and were excluded from the analysis. After excluding these patients, a total of 605 patients (Seo-gu: 290, Gokseong-gun: 315) participated in the study.
The study protocol was approved by the Institutional Review Board of Chonnam National University Hospital, and informed consent was obtained from each subject.
II. Methods
A well-trained examiner interviewed the patients using a questionnaire that included items on smoking status, diabetic duration, anti-diabetic medication use, antihypertension medication use, and a history of past illnesses.
Anthropometric measurements, including weight, height, and waist measurements, were obtained using standard techniques. The body mass index (BMI) was calculated using the following formula: weight (kg)/height2 (m2). Blood pressure was measured on the right upper arm using a mercury sphygmomanometer (Baumanometer; WA Baum Co., Inc., Copiague, NY, USA) with an appropriately sized cuff after subjects had rested at least 10 min in a sitting position. The first appearance (phase I) and disappearance (phase V) of Korotkoff sounds were used to define systolic and diastolic blood pressure, which was read to the nearest 2 mmHg. Two consecutive measurements of systolic and diastolic blood pressure were taken in 5 minutes intervals, and the average was used in the analysis.
All laboratory specimens were taken following overnight fasting. The serum was separated onsite and stored at -70℃ until further analysis. All serum samples were examined using an automatic analyzer (Hitachi-7600, Hitachi Ltd, Tokyo, Japan). HbA1c was measured by high-performance liquid chromatography (HPLC) using the Variant II (Bio-Rad, Hercules, CA, USA). Total cholesterol, high-density lipoprotein (HDL) cholesterol, and triglyceride concentrations were analyzed using enzymatic methods. Low-density lipoprotein (LDL) cholesterol was estimated as proposed by Friedewald et al. [25], except when triglyceride levels exceeded 400 mg/dL. In such instances, the data were treated as missing.
Kidney function was assessed using the estimated glomerular filtration rate (eGFR), which was calculated by the Modification of Diet in Renal Disease (MDRD) formula as follows [26]: 186.3 × (serum creatinine-1.154) × (age-0.203) × 0.742 (if female), with the serum creatinine concentration expressed in mg/dL.
Non-mydriatic digital images of the retina of each eye were taken using a retinal camera (TRC-NW200, Topcon Co., Japan). Retinopathy was considered present if any characteristic lesions as defined by the Early Treatment Diabetic Retinopathy Study (ETDRS) severity scale were present (microaneurysms, hemorrhages, cotton wool spots, intraretinal microvascular abnormalities, hard exudates, and new retinal vessels). If the ETDRS severity score was 10, the patient was defined as having diabetic retinopathy [27].
The CCA-IMT and the presence of carotid plaques were assessed by physicians using high-resolution mode B ultrasonographic scans of the carotid arteries (SonoAce 9900, Medison, Korea) with an electrical linear array transducer (7.5 MHz). The CCA-IMT was defined as the distance from the leading edge of the first echogenic line to the second echogenic line, which indicated the media-adventitia interface. Images of the thickest point within 10 mm from the carotid bulb to the CCA were saved as CCA-IMT, and then measured using SigmaScan Pro version 5.0.0 (Systat Software Inc., Chicago, IL, USA).
The examiners evaluated the CCA, carotid bulb, and internal carotid artery to determine the levels of carotid plaque. Protrusions into the lumen that were 100% thicker than the nearest area were defined as plaques. If the plaque was the thickest point, the distance to the nearest point without plaques was defined as the CCA-IMT. The presence of carotid plaques was recorded if at least one lesion was detected in six segments.
After a minimum 5 minutes rest period, the baPWV and ABI were calculated automatically in the supine position using the VP-1000 system (Colin Co., Komaki, Japan) with cuffs around both arms and ankles. If any of the ABIs were < 0.9, the patient was defined as having PAD.
III. Statistical Analyses
Data are provided as the mean ± standard deviation (SD) for continuous variables, the percentage for categorical variables, and the median (interquartile range) for triglyceride and baPWV. An independent sample t-test or Mann-Whitney U test was used to examine significant differences in the mean values between the two groups, and a chi-square test was used to examine differences in the frequency. The associations between CCA-IMT, baPWV, and clinical characteristics were examined using partial correlation, controlling for age and sex. To evaluate the association between the surrogate of macroangiopathy (CCA-IMT, baPWV, carotid plaque, and PAD) and diabetic retinopathy, different models were constructed using multiple logistic regression analyses by adjusting for confounding risk variables (age, sex, duration of diabetes, HbA1c, total cholesterol (TC), log-transformed triglycerides, HDL cholesterol, eGFR, BMI, and history of hypertension). These confounding risk variables were selected through a literature review [22-24]. Because there is no clear cut-point for categorization, CCA-IMT and baPWV were divided into tertiles: CCA-IMT, 0.39 to 0.65 mm, 0.66 to 0.78 mm, and 0.79 to 1.30 mm; baPWV, 9.9 to 15.8 m/s, 15.9 to 18.9 m/s, and 19.0 to 38.0 m/s.
Statistical analyses were performed with the SPSS version 19.0 (SPSS Inc, Chicago, IL, USA). A level of p < 0.05 was accepted as statistically significant.
RESULTS
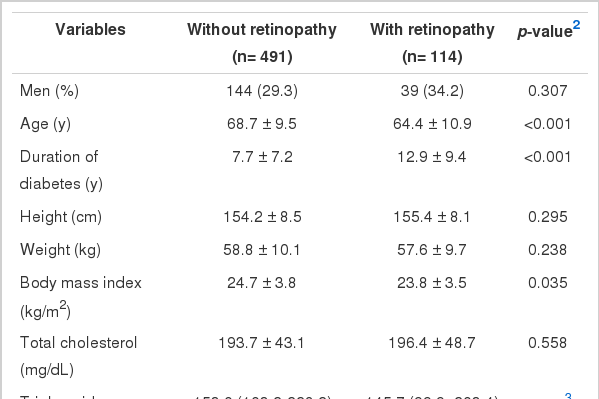
Table 1 summarizes the general characteristics of the study groups with and without retinopathy. The presence of diabetic retinopathy was confirmed in 114 (18.8%) of the 605 patients who were eligible to take part in the study. The mean age of the subjects with diabetic retinopathy was significantly lower compared to subjects without diabetic retinopathy. In addition, the response rate of the Gokseong-gun study (64.3%) was higher than that of the Seo-gu study (48.0%), and a significant mean age difference existed between the two regions. However, the mean age of the subjects with diabetic retinopathy was significantly lower compared to subjects without diabetic retinopathy in each of the two regions. The stratified analysis also showed that there was no evidence of effect modification by region (data not shown). The mean values of HbA1c and the duration of diabetes were significantly higher among subjects with diabetic retinopathy compared to subjects without diabetic retinopathy. The baPWV was higher in subjects with diabetic retinopathy, but this was not statistically significant.
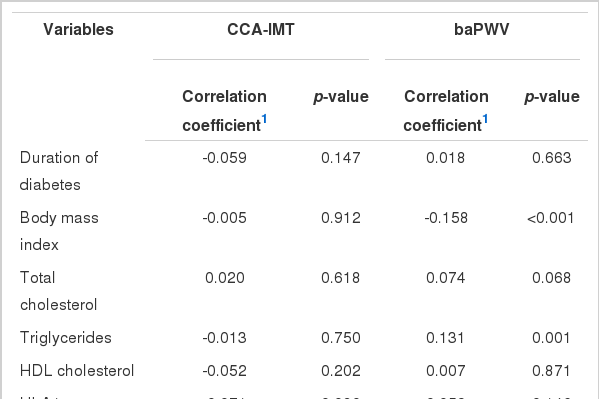
The associations between CCA-IMT, baPWV, and clinical characteristics are presented in Table 2. The CCA-IMT was not correlated with clinical characteristics, while baPWV was significantly correlated with BMI (r=-0.158, p<0.001) and triglycerides (r=0.131, p=0.001).
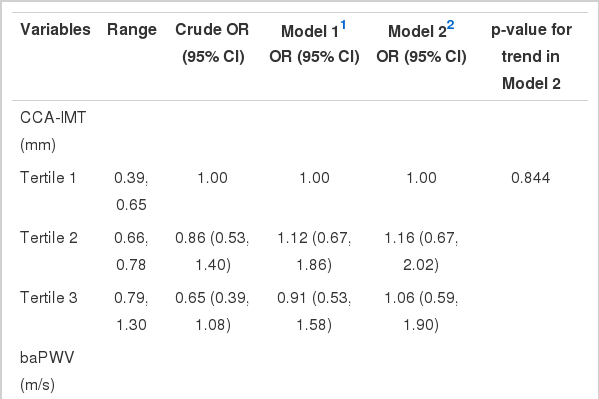
The odds ratios (ORs) for diabetic retinopathy according to the CCA-IMT levels are listed in Table 3. When adjusted for other covariates (i.e., sex, age, duration of diabetes, HbA1c, TC, log-transformed triglycerides (logTG), HDL, eGFR, BMI, and history of HTN), the CCA-IMT was not significantly associated with diabetic retinopathy.
The ORs for diabetic retinopathy according to baPWV levels are listed in Table 3. Compared to the first tertile, crude ORs (95% confidence interval [CI]) for retinopathy were 1.45 (0.85 to 2.47) for the second tertile and 1.93 (1.16 to 3.24) for the third tertile. Adjustment for age and sex increased ORs (95% CI) for retinopathy: 2.48 (1.37 to 4.49) for the second tertile and 4.44 (2.38 to 8.31) for the third tertile. Further adjustment for other covariates (Model 2) did not change the association.
The ORs for diabetic retinopathy according to carotid plaques and PAD are listed in Table 3. When adjusted for all covariates (Model 2), the carotid plaques and PAD were not significantly associated with diabetic retinopathy.
DISCUSSION
The purpose of this study was to determine the association between macroangiopathy (CCA-IMT, baPWV, carotid plaque, and PAD) and diabetic retinopathy. We found that baPWV is significantly associated with diabetic retinopathy, whereas CCA-IMT, carotid plaque, and PAD are not.
There was no significant association between CCA-IMT and diabetic retinopathy among type 2 diabetic patients after adjusting for cardiovascular risk factors. These results are consistent with the findings of the Cardiovascular Health Study (CHS) [21]. However, the Chennai Urban Rural Epidemiology Study (CURES-2) and the Atherosclerosis Risk In Communities (ARIC) study reported a positive relationship between CCA-IMT and diabetic retinopathy [22,28]. We did not observe an independent association between carotid plaque, PAD, and diabetic retinopathy, whereas a positive relationship has previously been observed [20,29]. However, carotid plaque and PAD have been shown not to be significantly associated with diabetic retinopathy in some studies [30-32]. Selective survival may provide a potential explanation for these discrepant results. Severe atherosclerosis and retinopathy in diabetic patients were both associated with an increased risk of death, which may have resulted in individuals with these conditions being less likely to be examined than patients without these conditions [33]. The other potential reasons for the discrepant results may include different sample sizes, population characteristics, the lower magnification of our images, and the age distribution of the study population.
Arterial stiffness is a major contributor to CVD [34], and baPWV is correlated with the degree of atherosclerotic vascular damage [13,35]. The present study showed that baPWV has a distinct association with diabetic retinopathy contrary to other surrogates. The exact mechanisms underlying the association between atherosclerosis and diabetic retinopathy are poorly understood. In previous studies, analyses were conducted separately according to each surrogate [20,22-24,29,30]. An integrated theory to explain these observed phenomena has not been established. One possible explanation is that atherosclerosis and diabetic retinopathy share common risk factors in the causal pathway [17,18,20,22]. Although the adjustment for cardiovascular risk factors in our study did not change the association between surrogates of macroangiopathy and diabetic retinopathy, the possibility of an effect of unmeasured variables remained. In the present study, functional markers such as baPWV were associated with diabetic retinopathy, but structural markers such as IMT, carotid plaque, and PAD were not. This suggests that the association between macroangiopathy and microangiopathy may be due to a functional rather than structural process within the vascular system.
Interestingly, in the present study, without adjustment, there was no significant difference in the baPWV between subjects with diabetic retinopathy and subjects without diabetic retinopathy (p value=0.072). However, after adjustment for age and sex, baPWV was significantly associated with diabetic retinopathy. Therefore, this phenomenon may result from the positive confounding effect of age and sex.
This study has several limitations. First, this was a cross-sectional study. The association between macroangiopathy and diabetic retinopathy cannot be taken as a causal relationship. Nonetheless, we believe that the results may help shed light on the pathophysiological connections between the development and progression of macro- and microangiopathy in diabetes. Second, the study targets type 2 diabetic patients who were registered at a public health center in a certain area and does not represent the general population across the nation. Third, the mean age of the subjects with diabetic retinopathy was significantly lower than that of subjects without diabetic retinopathy. This phenomenon may be explained by selection bias due to either selective survival or nonparticipation (restricted movement). However, we cannot conclude that this phenomenon resulted from selection bias, because we did not compare general characteristics of study participants and nonparticipants. Fourth, we conducted multiple comparisons among the four phenotypes of macroangiopathy. However, after post-hoc multiple comparisons using the Bonferroni correction, these associations did not change substantially. Fifth, we used non-mydriatic color digital images of the retina. The accuracy of this method is slightly lower than the use of mydriatic color images. Despite these limitations, this study provides a valuable contribution, in that we evaluated the association between multiple phenotypes of macroangiopathy, including CCA-IMT, carotid plaque, PAD, and baPWV, and diabetic retinopathy in a single population.
In summary, baPWV is associated with diabetic retinopathy in type 2 diabetic patients, but CCA-IMT, carotid plaque, and PAD are not. Additional studies are required to further evaluate the common pathogenic mechanisms underlying these associations in Korean type 2 diabetic patients.
Notes
The authors have no conflicts of interest with the material presented in this paper.
This article is available at http://jpmph.org/.