The Effect of Breastfeeding Duration and Parity on the Risk of Epithelial Ovarian Cancer: A Systematic Review and Meta-analysis
Article information
Abstract
Objectives
We conducted a systematic review and meta-analysis to summarize current evidence regarding the association of parity and duration of breastfeeding with the risk of epithelial ovarian cancer (EOC).
Methods
A systematic search of relevant studies published by December 31, 2015 was performed in PubMed and EMBASE. A random-effect model was used to obtain the summary relative risks (RRs) and 95% confidence intervals (CIs).
Results
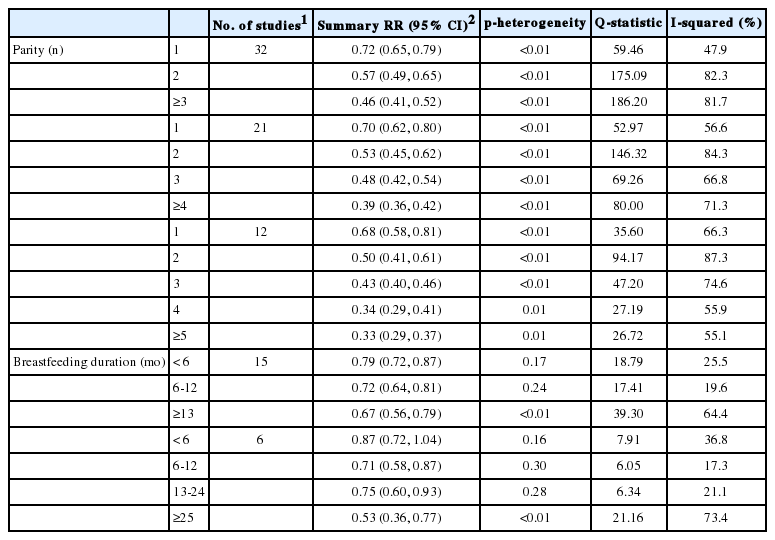
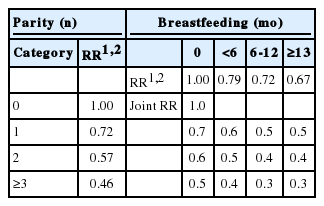
Thirty-two studies had parity categories of 1, 2, and ≥3. The summary RRs for EOC were 0.72 (95% CI, 0.65 to 0.79), 0.57 (95% CI, 0.49 to 0.65), and 0.46 (95% CI, 0.41 to 0.52), respectively. Small to moderate heterogeneity was observed for one birth (p<0.01; Q=59.46; I2=47.9%). Fifteen studies had breastfeeding categories of <6 months, 6-12 months, and >13 months. The summary RRs were 0.79 (95% CI, 0.72 to 0.87), 0.72 (95% CI, 0.64 to 0.81), and 0.67 (95% CI, 0.56 to 0.79), respectively. Only small heterogeneity was observed for <6 months of breastfeeding (p=0.17; Q=18.79, I2=25.5%). Compared to nulliparous women with no history of breastfeeding, the joint effects of two births and <6 months of breastfeeding resulted in a 0.5-fold reduced risk for EOC.
Conclusions
The first birth and breastfeeding for <6 months were associated with significant reductions in EOC risk.
INTRODUCTION
Worldwide, ovarian cancer is the seventh most common cancer in women. Furthermore, it is the sixth leading cause of cancer deaths in women and the second most common cause of death among those with gynecologic cancers [1]. Approximately 90% of ovarian cancers are of epithelial origin [2], with the remaining 10% composed of sex cord-stromal tumors (5% to 8%), germ cell tumors (3% to 5%), and other rare types of ovarian cancer [3].
Most ovarian cancers are life-threatening and are notorious for having a poor prognosis, as they are usually diagnosed at an advanced stage. Moreover, screening results based on pelvic imaging or tumor markers for early detection remain unsatisfactory [4]. Therefore, to reduce the risk of ovarian cancer, primary prevention, such as avoiding risk factors or strengthening exposure to preventive factors, is important.
Reproductive risk factors for epithelial ovarian cancer (EOC) have been extensively explored in epidemiologic studies. For instance, a pooled analysis of 12 US case-control studies in 1992 showed that parous women and those who had breastfed had a lower risk of EOC [5,6]. The protective effect of parity and breastfeeding against EOC is biologically plausible and can be explained by two hypotheses: (1) the incessant ovulation hypothesis, in which monthly ovulation might increase the odds of genetic mutations, potentially leading to subsequent malignant changes [7], and (2) the gonadotropin hypothesis, in which ovarian overstimulation by elevated gonadotropins might trigger hyperproliferation, including subsequent malignant transformation [8]. A pooled analysis in 1992 showed that the greatest protection was associated with the first birth and the first few months of breastfeeding [5]. However, this was only observed in a pooled analysis of six population-based case-control studies, not in hospital-based case-control studies.
Since 1992, many studies from around the world have reported associations of parity and breastfeeding with ovarian cancer. However, findings concerning the protective role of increasing parity and duration of breastfeeding remain inconsistent. For parity, some studies have indicated that the first birth reduces ovarian cancer risk more than subsequent births [9-13]. In contrast, other studies have reported that the second birth was associated with a greater protective effect [14-16]. Likewise, for breastfeeding, some studies have indicated that the first six months of breastfeeding reduce risk more than a month of subsequent breastfeeding [17,18], whereas other studies have reported that each additional six months of breastfeeding confer approximately the same level of additional risk reduction [12,19].
Therefore, we conducted a systematic review and meta-analysis to summarize the current evidence regarding the association of parity and duration of breastfeeding with EOC risk. The aim of this study was to clarify the threshold for risk reduction among the studies without heterogeneity across the results. An additional aim was to perform a meta-analysis to estimate the joint risk reductions associated with parity and breastfeeding.
METHODS
Search Strategy
We performed a literature search including studies published through December 2015 using the following search terms in the PubMed and EMBASE databases (1) (parity or “number of live births”) and (ovary or ovarian) and (cancer or tumor or neoplasm or malignancy) or (2) (breastfeeding or lactation) and (ovary or ovarian) and (cancer or tumor or neoplasm or malignancy). Furthermore, to find any additional published studies, a manual search was performed by checking all references of prior meta-analyses [5,6.8,20-23] and of all the original studies. This systematic review was planned, conducted, and reported in adherence to the standards of quality for reporting meta-analyses [24,25].
Study Selection
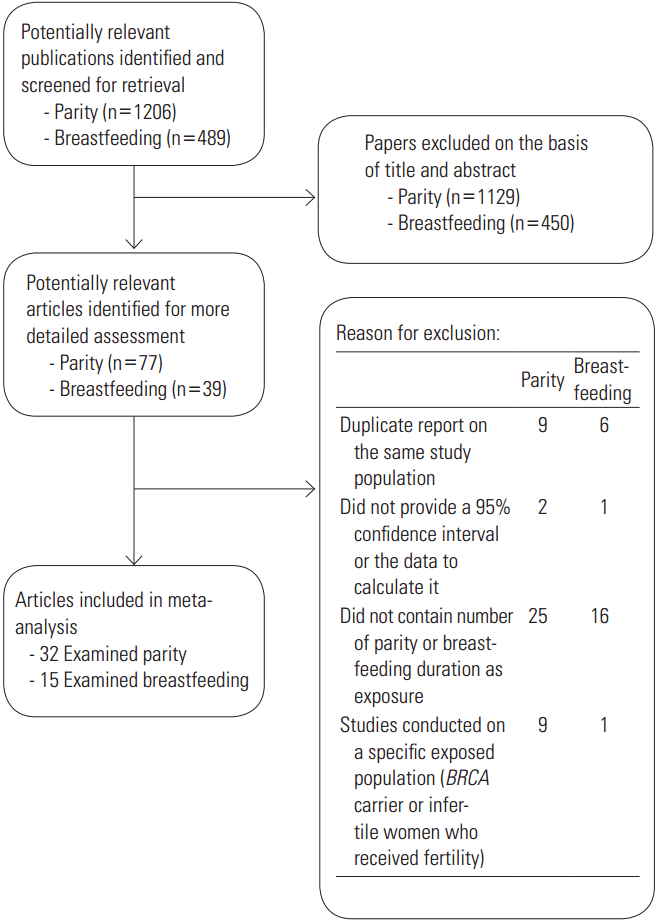
To be included, studies had to meet the following criteria: (1) the studies were observational (case-control or cohort studies), (2) the exposures of interest were the number of live births and the total duration of breastfeeding, (3) the outcome of interest was EOC, (4) odds ratios (ORs) or relative risk (RR) estimates with 95% confidence intervals (CIs) were reported or sufficient data were present to allow the calculation of these effect measures, and (5) articles were published in the English language. In the case of overlapping data, the study with the largest number of cases was included. As fertility treatments and BRCA mutation effects on EOC may alter the association between parity/breastfeeding and EOC [26], we excluded studies conducted on specific populations, such as BRCA-1 or BRCA-2 mutation carriers or infertile women treated with fertility drugs. The detailed steps of our literature search are shown in Figure 1.
Data Extraction
Data extraction was conducted independently by two authors. Disagreements were discussed and resolved by consensus. The following data were collected from each study: the first author’s last name, publication year, study region and design, study period, participant age, sample size (cases and controls or cohort size), exposure variables (parity or total breastfeeding duration), study-specific adjusted RR or OR with 95% CIs for each exposure category, and factors matched or adjusted for in the design or data analysis. If no adjusted RR or OR was presented, we included crude estimates. If no RRs or ORs were presented in a given study, we calculated them and the 95% CIs according to the raw frequencies presented in the article. The quality of the study was assessed independently by two authors using the 9-star Newcastle-Ottawa Scale (range, 0 to 9 stars) [27]. This measure assesses aspects of methodology in observational studies related to study quality, including the selection of cases, comparability of populations, and ascertainment of exposure to risks.
Statistical Analysis
The study-specific RRs or ORs with 95% CIs were used to determine the principal outcome. Because the OR closely approximates the RR for rare diseases, the RR can be estimated from a case-control study using the OR as an approximation [28]. Ovarian cancer is relatively rare and its absolute risk is low, with an incidence of 6.1 per 100 000 women [1]. Therefore, in the meta-analysis, ORs from case-control studies were used as an equivalent of RRs from cohort studies; we reported all results as RRs [22.29]. Mantel-Haenszel crude estimates of the RRs and corresponding 95% CIs were calculated when the RRs were not present but enough data were available. Logit RR estimates were calculated when the data were sparse. A random-effect model was used to obtain the summary RR and 95% CI. To assess whether the risk of EOC decreased with increasing parity or duration of breastfeeding, we categorized parity (1, 2, or ≥3; 1, 2, 3, or ≥4; and 1, 2, 3, 4, or ≥5) relative to nulliparity and total breastfeeding duration (<6 months, 6-12 months, or ≥13 months; and <6 months, 6-12 months, 13-24 months, or ≥25 months) relative to never having breastfed, as reported by most of the studies.
One study did not provide the required risk estimates for analysis or separate the risk estimates for different categories of parity or breastfeeding duration. For this study, we used the method proposed by Fleiss and Gross [30]. This method allows adjusted effect estimates and CIs to be calculated for any alternative comparison of levels and can help in a dose-response meta-analysis. Briefly, we combined risk estimates obtained through a simple fixed-effects meta-analysis wherein the subjects were divided into unexposed groups (i=0) and exposed groups (i=1, …, n), and estimates (Ri) with lower and upper 95% CIs were available. To obtain the R1+, we meta-analyzed R1, R2, R3, …, Rn using a fixed-effect model. The categories of parity or breastfeeding duration varied across studies; accordingly, the number of studies included in each meta-analysis and the summary RRs in each meta-analysis were different depending upon the number of categories.
Statistical heterogeneity among studies was evaluated with the Cochran Q and I-squared statistics [31]. The significance level for the Q statistic was defined as p-value<0.1. The I-squared value represents the proportion of total variation composed of between-study variation [31]. I-squared values ≤25%, 25.1-50%, 50.1-75%, and >75% were interpreted as indicating no, small, moderate, and significant heterogeneity, respectively [32]. Subgroup analyses were conducted to explore the potential sources of heterogeneity, according to the following characteristics: (1) study design (cohort, case-control studies); (2) the quality of study methodology across studies, with studies with ≥8 stars considered high-quality and those with ≤7 stars considered low-quality as per the 9-star Newcastle-Ottawa Scale; and (3) year of publication (<2000, ≥2000), respectively.
Publication bias was evaluated using the Begg rank correlation and the Egger linear regression test, in which p-vlaue <0.05 were considered representative of statistically significant publication bias [33].
From the meta-analyzed result, to calculate the RR for the joint effect of parity and breastfeeding, we applied the log-linear dose-response model proposed by Berlin et al. [34].
We configured the following formula for the multivariate linear logit regression of two factors:
Logit P=α+β1χ1+β2χ2;
where P is the probability of a particular outcome (EOC risk), α is the intercept from the linear regression equation, β is the regression coefficient multiplied by some value of the predictor, and χ is the risk factor (parity and breastfeeding).
Using this equation yields the value of the RR for the joint effects of parity and breastfeeding duration. For example, in the case of a subject who has no risk factors, logit(P) is α. In this case, the probability of EOC is exp(α)=1.0. In the case of a subject with only χ1, logit(P) is α+β1. In the case of a subject with both χ1 and χ2, logit(P) is α+β1+β2. Accordingly, the probability of EOC is exp(β1+β2)=OR1×OR2.
Since the category of parity and breastfeeding duration varied across studies, to calculate the RR for the joint effect of parity and breastfeeding, we used the summary RR for parity and breastfeeding duration that contained the largest number of studies.
All statistical analyses were performed with Stata version 12.0 (StataCorp., College Station, TX, USA).
RESULTS
Study Characteristics
The characteristics of the 32 studies included with data regarding parity and the 15 studies included with data regarding breastfeeding are shown in Supplemental Tables 1 and 2. For parity, six cohort studies and 26 case-control studies were included. The included studies were conducted between 1973 and 2008. Of the 32 studies, 14 were performed in North America, 12 in Europe, four in Asia, one in Australia, and one in Africa. For breastfeeding, two cohort studies and 13 case-control studies were included. The included studies were conducted between 1978 and 2008. Of the 15 studies, seven were performed in North America, six in Europe, one in Asia, and one in Australia.
Parity and Epithelial Ovarian Cancer Risk
Thirty-two studies had parity categories of 1, 2, and ≥3. The summary RRs for the first, second, and third births were 0.72 (95% CI, 0.65 to 0.79), 0.57 (95% CI, 0.49 to 0.65), and 0.46 (95% CI, 0.41 to 0.52), respectively (Table 1). Small to moderate heterogeneity was observed for the first birth (p<0.01; Q=59.46, I2=47.9%), whereas significant heterogeneity was observed for the second (p<0.01; Q=175.09; I2=82.3%) and third (p<0.01; Q=186.20; I2=81.7%) births. Analyses gave no indication of publication bias. Similar results were also observed for parity categories of 1, 2, 3, and ≥4 and 1, 2, 3, 4, and ≥5.
Duration of Breastfeeding and Epithelial Ovarian Cancer Risk
Fifteen studies had breastfeeding categories of <6 months, 6-12 months, and ≥13 months. The summary RRs for these categories were 0.79 (95% CI, 0.72 to 0.87), 0.72 (95% CI, 0.64 to 0.81) and 0.67 (95% CI, 0.56 to 0.79), respectively (Table 1). Small or no heterogeneity was observed for <6 months (p=0.17; Q=18.79; I2=25.5%) and 6-12 months (p=0.24; Q=17.41; I2=19.6%), whereas moderate heterogeneity was observed for ≥13 months (p<0.01; Q=39.30; I2=64.4%). Analyses gave no indication of publication bias. Similar results were also observed for the breastfeeding categories of <6 months, 6-12 months, 13-24 months, and ≥25 months.
Subgroup Analysis According to Study Design, Study Quality, and Publication Year
The results from the subgroup analysis according to study design, study quality, and publication year are shown in Table 2. In high-quality studies, the summary RRs for the first, second, and third births were 0.73 (95% CI, 0.64 to 0.84), 0.60 (95% CI, 0.49 to 0.74), and 0.46 (95% CI, 0.41 to 0.52), respectively. The summary RRs for <6 months, 6-12 months, and ≥13 months of breastfeeding were 0.79 (95% CI, 0.68 to 0.91), 0.82 (95% CI, 0.69 to 0.97), and 0.79 (95% CI, 0.66 to 0.95), respectively. The summary RRs of the first birth and <6 months of breastfeeding from the analysis of high-quality studies were almost identical to the values from the analysis of all 32 and 15 studies, without heterogeneity (I2=0%).
The summary RR for the first birth in cohort studies was weaker, and only had a borderline significant effect on EOC risk (RR, 0.86; 95% CI, 0.75 to 1.00) relative to case-control studies (RR, 0.69; 95% CI, 0.61 to 0.77). In contrast, with regards to breastfeeding, the summary RRs for <6 months were similar between cohort studies and case-control studies (Table 2), with small heterogeneity.
Relative Risk for the Joint Effect of Parity and Breastfeeding
The RR for the joint effect of parity and breastfeeding, obtained using the summary RR from the analysis of 32 studies with parity categories of 1, 2, and ≥3 and 15 studies with breastfeeding categories of <6 months, 6-12 months, and ≥13 months, is shown in Table 3. Compared to nulliparous women who never breastfed, uniparous women with no history of breastfeeding had a nearly 30% reduced risk for EOC (Table 3). Without breastfeeding, two births and three or more births elicited 40% and 50% reduced risks for EOC, respectively. When breastfeeding <6 months was added to each parity category, an additional 10% reduction in EOC risk was found.
DISCUSSION
The findings of this meta-analysis indicate that parity and breastfeeding experiences in women can help prevent EOC, which is typically life-threatening and has a poor prognosis. In particular, the first birth and the first six months of breastfeeding had a greater protective effect than did subsequent births and/or additional breastfeeding, although multiparity and additional breastfeeding did provide some additional protection. The risk reduction effect of the first birth on EOC risk was almost 30%, and the combined effect of the first birth and <6 months of breastfeeding was 40%; thus, breastfeeding provided a nearly 10% greater risk reduction. In regards to parity, the EOC risk reduction was highest for the first birth, with some additional protection from the second birth. However, slightly less risk reduction was observed for the third birth (Figure 2). Although a prior meta-analysis suggested a continuous risk reduction of approximately 14% for each additional pregnancy after the first [5], the current findings show different results, with a gradually decreasing risk from additional parity and/or breastfeeding duration that eventually plateaus (Table 1).
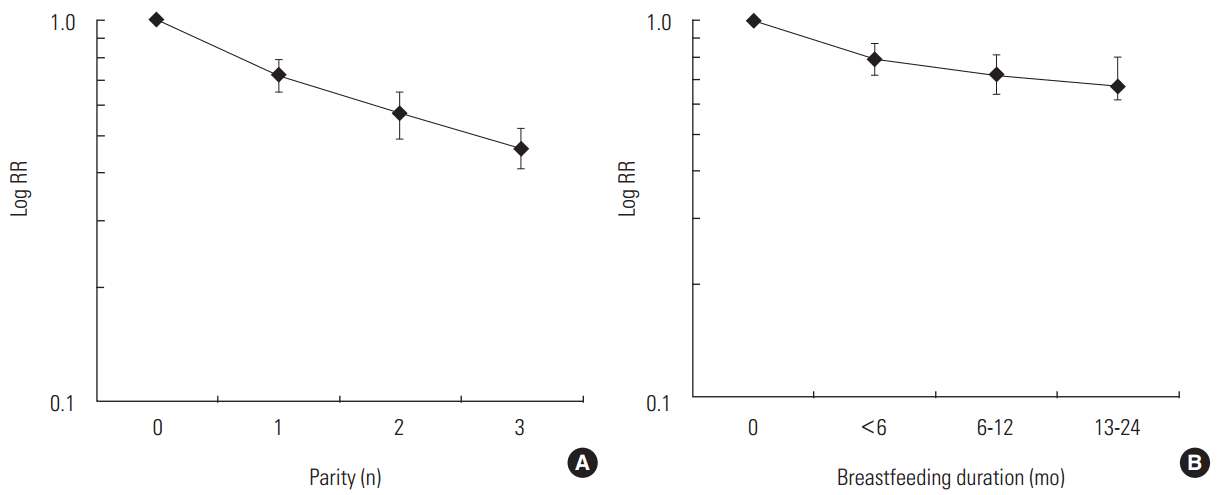
Decreasing epithelial ovarian cancer (EOC) risk with increasing parity and breastfeeding duration. (A) Decreasing EOC risk with increasing parity1,2. (B) Decreasing EOC risk with increasing breastfeeding duration1,2. 1The relative risks (RRs) in each category were estimated using a random effect model. 2We used summary RRs from 32 studies for parity and 15 studies for breastfeeding (shown in Table 1).
Pregnancy and breastfeeding are thought to reduce EOC risk by decreasing pituitary gonadotropin levels and inducing anovulation [7,35]. Pregnancy and breastfeeding are expected to decrease the likelihood of spontaneous genetic mutation under the incessant ovulation hypothesis and of the hyperproliferation of inclusion cysts under the gonadotropin hypothesis. However, the observation that multiparity and additional breastfeeding did not provide an equal amount of protection does not provide evidence for either of these hypotheses. Nevertheless, the results of two experimental studies provide biological evidence for the relatively weaker protective effect of additional parity and breastfeeding [36,37]. For instance, high progesterone levels during pregnancy can increase apoptosis, which may clear transformed cells from the ovarian epithelium, meaning that all the accumulated transformed cells are washed fully out by the first pregnancy. Therefore, the first pregnancy provides a stronger protective effect than subsequent pregnancies [36]. In regards to breastfeeding, breastfeeding in the first few months completely inhibits the pulsatile secretion of gonadotropin-releasing hormone and luteinizing hormone, leading to suppression of ovulation [37]. After a couple of months, ovulatory activity may return, even though breastfeeding continues [37]; thus, a longer duration of breastfeeding does not provide an additional protective effect.
Our finding of decreased EOC risk with longer breastfeeding is similar to that reported by prior meta-analyses in 2013 and 2014 [22,23], but differs from that of a meta-analysis of nine case-control studies conducted in developed countries in 2001, in which breastfeeding for ≥12 months was associated with a significant 0.72-fold reduced risk of EOC compared to never having breastfed, while breastfeeding <12 months did not show such an association (OR, 0.95; 95% CI, 0.80 to 1.12) [21].
Based on the RR for the joint effect of parity and breastfeeding, women who had two births and breastfed for <6 months had a 0.5-fold reduced risk of EOC. Our findings may provide evidence for developing guidelines for EOC prevention.
The strength of this meta-analysis is that it included all available studies, and the large number of EOC cases allowed for the investigation of the risk associated with different categories of parity and breastfeeding duration. However, the current study also has several limitations. First, our meta-analysis was restricted to studies published in indexed journals and might not have included unpublished studies. Second, some residual confounders may not have been excluded and may have influenced the protective effect of parity and breastfeeding, although a large number of potential confounding factors, such as age, race, and use of oral contraceptives, were adjusted for in most of the included studies. Third, significant heterogeneity must be considered. Significant heterogeneity was present in the analysis assessing whether the risk of EOC decreased with increasing parity or duration of breastfeeding, especially for higher parity and longer duration of breastfeeding. Despite the subgroup analyses, heterogeneity still existed in the results of the highest category of parity and longest duration of breastfeeding. Categories of parity and duration of breastfeeding, especially the highest parity and longest duration of breastfeeding, differed among studies and may have contributed to the heterogeneity in the results. However, the first birth and <6 months of breastfeeding showed little heterogeneity, and similar results were found in the subgroup analysis to those of the high-quality studies. Fourth, as a meta-analysis of observational studies, the data were prone to biases such as recall and selection bias inherent in the original studies. Cohort studies are less susceptible to bias than case-control studies because information is collected before the diagnosis of the disease. However, only a small number of cohort studies have been published; therefore, it was not possible to analyze the various categories of parity and breastfeeding duration using cohort studies alone. Fifth, the included studies were primarily from North America and Europe. Therefore, the findings may not apply to Asian populations with a low incidence of ovarian cancer.
CONCLUSION
In conclusion, our findings indicated that the first birth and breastfeeding for <6 months were associated with significant reductions in EOC risk. As a modifiable reproductive risk factor, two childbirths and additional breastfeeding, regardless of breastfeeding duration, can reduce EOC risk by 50%. These findings may help to generate recommendations for the prevention of EOC.
ACKNOWLEDGEMENTS
This work was supported by a grant from the National R&D Program for Cancer Control, Ministry for Health, Welfare and Family Affairs, Republic of Korea (0920010).
Notes
CONFLICT OF INTEREST
The authors have no conflicts of interest associated with the material presented in this paper.
SUPPLEMENTAL MATERIAL
Details of studies on parity and ovarian cancer risk
Details of studies on breastfeeding and ovarian cancer risk