Hepatitis B, C, and D Virus Infections and AFP Tumor Marker Prevalence Among the Elderly Population in Mongolia: A Nationwide Survey
Article information
Abstract
Objectives
Infections with hepatitis B, C, and D virus (HBV, HCV, and HDV) are a major public health problem and lead to serious complications such as cirrhosis and hepatocellular carcinoma. We aimed to determine the seroprevalence of hepatitis B surface antigen (HBsAg), anti-HCV, anti-HDV immunoglobulin G, alpha-fetoprotein (AFP), and dual and triple hepatitis virus infections in Mongolia.
Methods
A total of 2313 participants from urban and rural regions were randomly recruited for this cross-sectional study. A questionnaire was used to identify the risk factors for hepatitis virus infections, and the seromarkers were measured using immunoassay kits.
Results
Among all participants, the prevalence of HBV, HCV, and HDV was 15.6%, 36.6%, and 14.3%, respectively. The infection rates were significantly higher in females and participants with a lower education level, rural residence, older age, and a history of blood transfusion. HBV and HCV co-infection was found in 120 (5.2%) participants and HBV, HCV, and HDV triple infection was detected in 67 (2.9%) participants. The prevalence of elevated AFP was 2.7%, 5.5%, and 2.6% higher in participants who were seropositive for HBsAg (p=0.01), anti-HCV (p<0.001), and anti-HDV (p=0.022), respectively. Elevated AFP was more prevalent in participants co-infected with HBV and HCV (5.8%, p=0.023), HBV and HDV (6.0%, p<0.001), and triple-infected with HBV, HCV, and HDV (7.5%) than in uninfected individuals.
Conclusions
Nearly half (49.8%) of the study population aged ≥40 years were infected with HBV, HCV, or HDV, and 22.4% had dual or triple infections.
INTRODUCTION
Viral hepatitis and its complications, such as cirrhosis and liver cancer, are a major public health issue worldwide, especially in Mongolia. Liver cancer is the second leading cause of death in Mongolia [1,2] and the most common cancer in both sexes, with estimated rates of 114.7 and 74.6 per 100 000 males and females, respectively [2].
Hepatitis and its complications are most commonly caused by the 5 hepatotropic viruses, A, B, C, D, and E. Globally, there are approximately 257 million people with hepatitis B virus (HBV) infection and 71 million people with chronic hepatitis C virus (HCV) infection, with the highest prevalence in Asia, especially in the Western Pacific region [3–5]. Although some countries do not report the prevalence of hepatitis D virus (HDV) infections, it is estimated that 15–20 million additional people worldwide are living with HDV infection.
HBV, HCV, and HDV are blood-borne viruses transmitted by blood or body fluids that contain blood [6,7]. HBV is most commonly transmitted during childbirth, unprotected sexual intercourse, and exposure to saliva, menstrual, vaginal, and seminal fluids [8]. In contrast, HCV is usually transmitted parenterally, by intravenous drug use or transfusion of unscreened blood and blood products, whereas transmission during sexual contact or childbirth is much less common [9]. HDV infection requires HBV for replication, and therefore occurs only in patients infected with HBV [10]. HDV is transmitted through blood and body fluids, and approximately 5% of people with chronic HBV infection are co-infected with HDV [11].
Chronic infections with HBV and HCV are often asymptomatic, and most infected individuals are unaware of their status until significant liver damage has occurred. Severe liver disease is more frequent when patients are co-infected with both viruses [12]. HBV and HDV co-infection has also been associated with the rapid progression of liver disease. Hepatocellular carcinoma is the most severe hepatitis complication and can be diagnosed early with alpha-fetoprotein (AFP) testing [13].
The prevalence of hepatitis varies widely around the world. In developed countries, the prevalence is approximately 0.1%, whereas in developing countries it can range from 3% to 20% or higher [14]. HBV infection has decreased in the Mongolian population since 1991 with the advent of a vaccination program and the introduction of disposable syringes [1]. However, this decline is seen in the younger population, and HBV and associated disease persist in the middle-aged and elderly population [15]. Furthermore, financial barriers to testing and treatment, as well as low hepatitis screening and treatment rates, are significant issues [16].
Recent studies indicate that 19.4% of the adult population in Mongolia is infected with either HBV or HCV. The highest prevalence of HCV (25.8%) was observed in older age groups, while there were no significant differences among age groups for HBV [15]. The seroprevalence of HDV among school children was reported to be 6.1% in 2006 [17], and the seroprevalence in adults aged 20–70 was 7.2% in a 2015 study [18]. However, no studies have investigated the status of triple infections and liver cancer markers in Mongolian adults. Therefore, this study aimed to assess the prevalence of HBV, HCV, HDV, and liver cancer markers among Mongolian adults aged ≥40 years.
METHODS
Study Design and Period
A population-based cross-sectional study was conducted in 2009 to determine the seroprevalence of hepatitis B surface antigen (HBsAg), anti-HCV and anti-HDV antibodies, associated risk factors, and AFP status among Mongolian adults aged ≥40 years. People aged ≥40 years were included from selected provinces, rural soums, and districts representing 4 major regions of the country, including the city of Ulaanbaatar. The sample frame was designed to enable statistically representative estimates of the prevalence of hepatitis virus infection at the national level.
Study Variables
Dependent variables
The dependent variables were serostatus of HBV, HCV, HDV, and AFP serostatus.
Independent variables
Socio-demographic characteristics such as age, residential area, and education status were determined. Histories of blood transfusion, surgical procedures, dental operations, tattooing, unsafe injections, and other possible risk factors were investigated.
Study Sampling
A stratified, multi-staged, random sampling method was used to select the study population. Mongolia is divided into 4 major geographical regions: Central, Eastern, Western, and Khangai, as well as the capital, Ulaanbaatar. To obtain a demographically and geographically representative sample, 1 province from each region and several districts from the city were selected in the first stage. Administratively, provinces in Mongolia are divided into soums, while city districts are divided into khoroos. Therefore, in the second phase, 13 soums and khoroos were randomly selected from the provinces and districts, respectively. Finally, a list of the names of residents aged ≥40 years was prepared to randomly select participants. The study participants were then invited to their local health centers for questionnaire interviews and blood sample collection.
According to a previous study on the prevalence of HBV [19], the required sample size was calculated using 15% prevalence, and a minimum sample size of 1225 was obtained using the following formula:
n=sample size
e=margin of error (2%)
p=prevalence (15%)
z=critical value at 5% (1.96)
The inclusion criteria were age ≥40 years, Mongolian citizenship, provision of a signed informed consent form, and no diagnosis of liver fibrosis or liver carcinoma. All other participants were excluded.
Data Collection
Socio-demographic characteristics and potential risk factors
In total, 2313 people aged ≥40 years from selected provinces, rural soums, and districts representing the 4 regions of the country and the capital, Ulaanbaatar, were included. After providing informed consent, participants were asked to provide a blood sample for hepatitis B, C, D, and AFP assays and were interviewed directly. Each participant was asked about exposure to any of the following risk factors related to medical services: dental or gum treatment, intramuscular or intravenous injections in the hospital or at home, minor or major surgery, acupuncture, transfusion of blood and blood products, hemodialysis, and tattooing. Participants’ medical history of liver disease, hepatocellular carcinoma, cirrhosis, and liver fibrosis was evaluated by reviewing their health records at the health centers.
Blood sample collection and processing
A 10 mL specimen of venous blood was collected from each participant, centrifuged (2000–2500×g) to separate serum and blood cells, and stored at −80°C until tested.
Serological assay
HBsAg was analyzed by bioluminescent enzyme immunoassay (BLEIA) following the instructions of the BLEIA “Eiken” HBsAg kit (Eiken Chemical Co., Ltd., Tochigi, Japan) [20]. Anti-HCV analysis was done using the particle agglutination method (Ortho HCV Ab PA test II; Fujirebio Inc., Tokyo, Japan). Anti-HDV analysis was done by in-house enzyme-linked immunosorbent assay (ELISA), using purified recombinant S-HDAg protein that had been expressed in the pupae of a silkworm, as described by Inoue et al. [21]. AFP was determined using in-house ELISA according to the method described by Nomura et al. [22] with the following modifications: Wells of an Immunoplate (Greiner 762071; Greiner Bio-One GmbH, Frickenhausen, Germany) were coated with monoclonal anti-AFP (No. 2051) and post coated with 40% (v/v) fetal calf serum (FCS). The test serum (50 μL) was delivered to each well and individual wells received 50 μL of phosphate-buffered saline supplemented with 25% FCS and 2% (v/v) normal mouse serum (S25–100 mL: Merck KgaA, Darmstadt, Germany) and containing monoclonal anti-AFP (No. 2062) that had been linked to horseradish peroxidase. The plate was incubated at 25°C for 1 hour and washed with washing solution (saline with 0.05% Tween 20). Next, 50 μL of BioFX TMB Reagent (Surmodics, Eden Prairie, MN, USA) were delivered to each well, and the plate was incubated at 25°C for 1 hour. The reaction was terminated by adding BioFX Stop Reagent (Surmodics), and the intensity of developed color was determined by the absorbance at 450 nm. The specificity of this assay was verified by absorption with goat antiserum to human AFP (MP Biomedicals, Santa Ana, CA, USA). Samples with optical density values equal to or greater than those corresponding to 10 ng/mL of AFP were considered positive in this assay.
All assays were conducted at the laboratory of the Division of Virology, Department of Infection and Immunity, Jichi Medical University, Japan.
Statistical Analysis
Statistical analyses were performed using SPSS version 22.0 (IBM Corp., Armonk, NY, USA). Socio-demographic and risk factor variables were analyzed by the Pearson chi-square test or the Fisher exact test. To determine the associations between hepatitis B, C, and D infections and the risk factors, univariate and multivariate binary logistic regression analyses and odds ratio (OR) calculations were used. A level of p<0.05 was considered to indicate statistical significance.
Ethics Statement
Consent was obtained from each participant. The study was reviewed and approved by the Ethics Committee of Research at the Mongolian National University of Medical Sciences on January 20, 2009 (protocol No. 4).
RESULTS
Socio-demographic Characteristics of Study Participants
A total of 2313 participants from 4 regions of Mongolia and the city of Ulaanbaatar were included in the study. We ensured representative sampling from each age group, with 467 (20.1%), 518 (22.3%), 500 (21.6%) and 828 (35.5%) participants from the 40–44 years, 45–49 years, 50–54 years, and ≥55 year age groups, respectively. Regarding residential area, 1289 (55.7%) participants were from Ulaanbaatar (urban) and 1024 (44.3%) were from province centers (n=328) and soums (n=696) (Table 1).
Hepatitis B Virus, Hepatitis C Virus, Hepatitis D Virus, and Alpha-fetoprotein Seroprevalence
The distribution of serological indicators according to the descriptive characteristics of the study group is shown in Table 1. HBsAg seropositivity was found in 361 (15.6%) of the participants, specifically in 149 (17.4%) male and in 212 (14.6%) female (p=0.074). No statistically significant differences were found in HBsAg between age groups (p=0.107) and education level (p=0.518). However, significant differences in HBsAg prevalence were seen between urban (14.0%), province (11.9%), and soum (20.4%) residents with the highest prevalence in the soum residents (p<0.001).
Anti-HCV positive results were detected in 846 (36.6%) participants. Significant differences were seen according to sex (p<0.001), with a higher prevalence in female (41.5%) than in male (28.2%). Serological status differed according to age group (p<0.001). Anti-HCV seropositivity increased with age, with the highest prevalence observed among people aged ≥55 years (47.1%). Regarding residential area, anti-HCV positivity was most common among soum residents (41.1%), followed by urban (35.2%) and province center residents (32.3%) (p=0.008). Statistically significant differences were also noted in terms of education level (p<0.001), with the highest prevalence among uneducated participants (58.7%).
Anti-HDV positive results were detected in 331 (14.3%) participants, including 158 (18.4%) male and 173 (11.9%) female (p<0.001). In addition, seropositivity was highest among soum residents (18.0%), followed by urban (12.6%) and province center residents (13.4%) (p=0.004).
High AFP results were detected in 88 (3.8%) participants, and a significant difference in prevalence was seen between female (4.5%) and male participants (2.7%) (p<0.05).
Table 2 shows the distribution of HBsAg, anti-HCV, anti-HDV immunoglobulin G (IgG), and AFP in relation to risk behavior. Among those who tested positive for HBsAg, a borderline significant difference was found between home injection users and non-users (p<0.05). Anti-HCV-positive participants were more likely to have a history of surgery (p=0.004), acupuncture treatment (p=0.004), blood transfusion (p<0.001), or injection at a hospital (p=0.006). No significant difference in risk behaviors was found among anti-HDV-positive participants. Use of injections at home (p=0.016) and blood transfusion in the past (p=0.002) were significant for elevated AFP levels. Overall, a positive anti-HCV serological test result was most prevalent among study participants for all indicators compared to HBsAg, anti-HDV IgG, and AFP.
Hepatitis B Virus, Hepatitis C Virus, Hepatitis D Virus Co-infection and Triple-infection Status
We assessed co-infection and triple infections with HBV, HCV, and HDV among study participants (Table 3). HBV and HCV co-infections were found in 120 (5.2%) participants. Significant differences were observed according to residential area (p<0.001), with the highest prevalence among soum residents (9.3%). Significant differences were also seen according to education status (p=0.015) with co-infections being more common among participants with a primary education (9.7%), followed by participants with a vocational education (6.6%) and uneducated participants (6.5%).
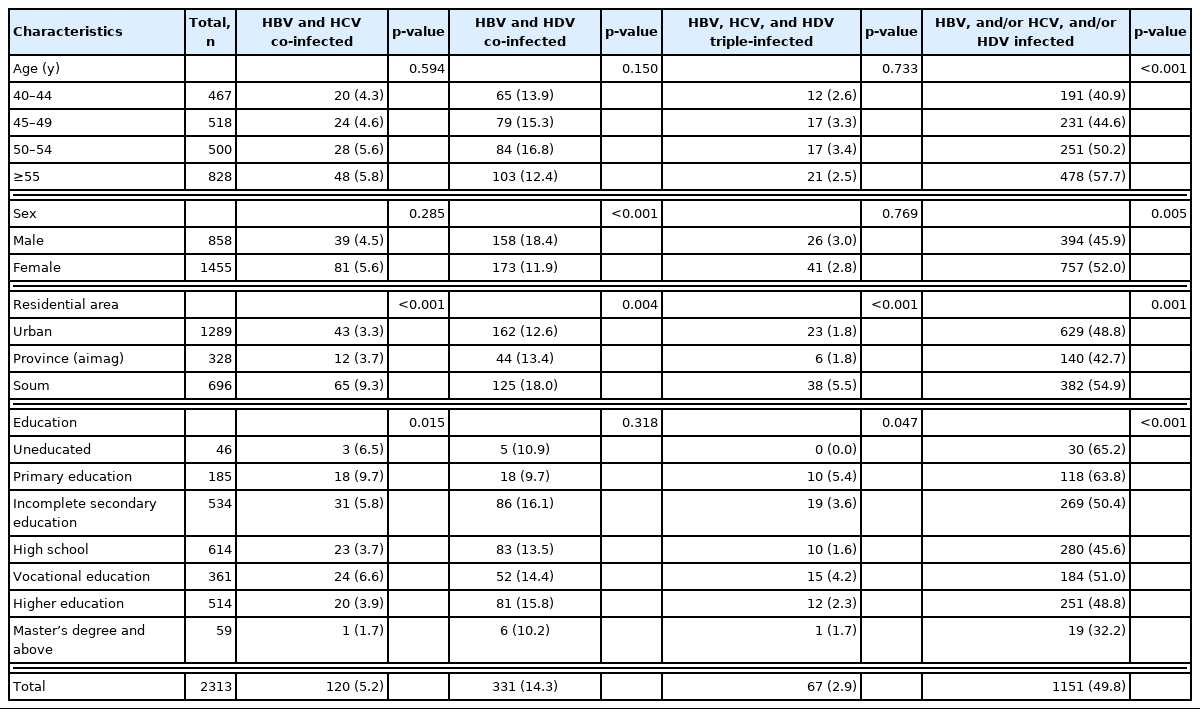
HBV, HCV, and/or HDV co-infection and triple-infection status in relation to socio-demographic characteristics
Co-infections with HBV and HDV were found in 331 (14.3%) participants. Significant differences in prevalence were seen between male (18.4%) and female (11.9%) participants (p<0.001) and by area of residence (p=0.004), with the highest prevalence in soum residents (18.0%).
The 67 (2.9%) participants who had triple infections showed significant differences in residential area (p<0.001), with the highest prevalence among soum residents (5.5%), and in education status (p=0.047), with 15 (4.2%) individuals reporting a vocational education.
Hepatitis B Virus and/or Hepatitis C Virus and/or Hepatitis D Virus Infection Status
In this study, 1151 (49.8%) participants were infected with either HBV, HCV, or HDV. We observed a higher prevalence of hepatitis virus infection in female (52.0%) than in male (p=0.005) and among participants who were ≥55 years of age (57.7%, p<0.001). In addition, hepatitis virus infection prevalence was higher among soum residents (54.9%, p=0.001) and the uneducated (65.2%, p<0.001) than those with urban residence and higher education.
Potential Risk Factors for Hepatitis B Virus, Hepatitis C Virus and Hepatitis D Virus Infections Among Study Subjects
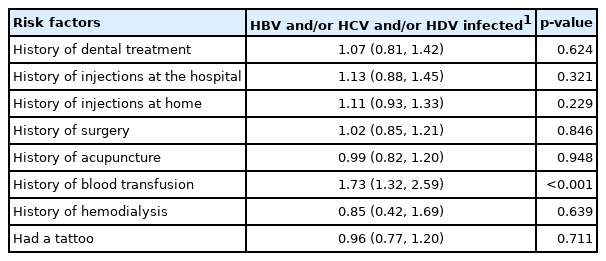
The associations between HBV, HCV, and HDV infections and risk factors were analyzed using binary logistic regression. Multinomial logistic regression analysis was used to control for covariates such as age, sex, and education level, as these socio-demographic characteristics differed significantly among seropositive markers in the chi-square analysis. The potential risk factors for HBV, HCV, and HDV infections among study participants are listed in Table 4. We found that a history of blood transfusion increased the risk of hepatitis infection by 1.73 times (95% confidence interval [CI], 1.32 to 2.59; p<0.001).
Alpha-fetoprotein Status in Relation to Hepatitis B Virus, Hepatitis C Virus, and Hepatitis D Virus Infection Status
The mean value (with standard deviation) of AFP levels was 0.76±0.87 ng/mL. AFP status relative to HBV, HCV, and HDV infection status is shown in Table 5. The prevalence of elevated AFP was 2.7%, 5.5% and 2.6% higher in participants who were seropositive for HBsAg (p=0.013), anti-HCV (p<0.001) and anti-HDV (p=0.022), respectively. Elevated AFP was more prevalent in participants co-infected with HBV and HCV (5.8%, p<0.001), HBV and HDV (6.0%, p<0.023), and triple-infected with HBV, HCV, and HDV (7.5%) than those not infected. Nearly 7% (n=78) of participants infected with either HBV, HCV, and HDV had elevated AFP levels (p<0.001).
DISCUSSION
Viral hepatitis infections and their complications are a major public health issue worldwide, especially in Mongolia. Previous studies have reported the prevalence of HBV and HCV among blood donors, healthy individuals, and the general Mongolian adult population, with results ranging from 7.8% to 11.0% for HBsAg and 11% to 25% for anti-HCV [15,23,24]. According to our study, the prevalence of HBV, HCV, and HDV in adults was 15.6%, 36.6%, and 14.3%, respectively. This is much higher than reported in other studies and can be explained by differences in sample size and study design. We included only adults aged 40 years and over, who constitute a high-risk population because vaccination and other infection-related public health interventions were not introduced until 1990.
In another study conducted by Davaalkham et al. [15] in 2006, the seroprevalence of HBV and HCV in the general Mongolian adult population (n=1158) was determined to be 11.1% and 8.5%, respectively. The prevalence of HCV was highest in older age groups (≥50 years) at 25.8%. Our study results were higher with the prevalence of HCV in the 50–54 years and ≥55 years age groups at 35.0% and 47.1%, respectively. This could be explained by new drug interventions introduced between our studies that showed a better survival rate.
The study conducted by Tserenpuntsag et al. [23] among blood donors (n=923) found seropositivity of HBsAg and anti-HCV in 7.8% and 9.6% of participants, respectively. HBsAg was more prevalent in donors 18–19 years, while anti-HCV was most common in donors aged ≥40 years.
Baatarkhuu et al. [19] reported a 14.7% HDV infection level in 624 patients diagnosed with acute hepatitis, which was similar to our findings.
The distribution of HBV, HCV, and HDV infections varies worldwide depending on the endemicity rate. The global prevalence of HBV infection in the general population was 3.5% in 2015 [11]. In the adult population over 30 years of age, the prevalence ranged from less than 5% [24] to approximately 10% [25]. According to our findings, the prevalence of HBV in Mongolia is much higher in the adult population aged ≥40 years than the global average.
The global prevalence of HCV was 1% in 2015 [11]. HCV prevalence in the adult population of the United States and the Czech Republic was 1.19% [26] and 1.67% [27], respectively. In comparison, the prevalence of HCV among Mongolian adults is very high.
According to recent studies from 61 countries, the overall prevalence of HDV was 0.98% (95% CI, 0.61 to 1.42) [28]. In comparison, the prevalence among Mongolian adults is again very high.
In a 2005 study by Tsatsralt-Od et al. [29] involving 207 patients with chronic liver disease, dual-infection of HBV/HDV and HBV/HCV was found in 26.6% and 7.7% of patients, respectively. In addition, triple infection was detected in 30% of patients. According to our results, co-infection with HBV/HDV, HBV/HCV, and triple infection were detected in 15.1%, 5.2%, and 5.5% of participants, respectively. Only patients with chronic liver disease participated in previous studies, whereas our study was conducted nationwide.
No statistically significant difference was found in the prevalence of HBV according to sex. However, a statistically significant higher rate of HCV was seen in female, and a higher prevalence of HDV and HBV/HDV co-infection was found among male. The binary logistic regression model showed that female had a higher risk of infection.
In terms of age group, no differences in HBV prevalence were found, but anti-HCV seropositivity increased progressively from the 40–44-year age group (25.9%) to those ≥55 years of age (47.1%). This trend is similar to those reported in other studies conducted in Mongolia [23] and Morocco [30]. Overall, increasing age increases the risk of infection with HBV, HCV, and HDV.
There were significant differences among the results for participants from urban areas, provinces, and soum areas. A higher prevalence of all virus types was found among rural residents, especially those living in soums. Similarly, a higher prevalence of co-infections and triple-infections was found among soum residents. Another study from 2004 to 2005 also reported that the prevalence of HCV tended to be higher among rural provincial residents [31].
Education status was shown to affect the prevalence of dual infections (HBV/HCV) and triple infections, with people who had a primary education, a vocational education, or were uneducated tending to be more seropositive. This may be explained by their socioeconomic status, as differences in income and education status have been previously associated with the prevalence of HCV and co-infection [32,33].
Each participant was asked about risk factor exposures such as dental treatments; injections in the hospital and at home; a history of surgery, acupuncture, blood transfusions, hemodialysis, or tattoos. We found a significant association between infection status and blood transfusion history. Likely, there are still high numbers of individuals in Mongolia infected with hepatitis through blood transfusion that are not aware of their status.
Previous studies have shown that mono-infections, co-infections, and triple infections promote faster progression of liver disease [34], and this was confirmed by our study. We found significantly higher levels of the hepatocellular carcinoma marker AFP among hepatitis-infected people. Co-triple-infection can lead to liver cancer, and this infection status had a strong impact on AFP levels in our study.
Most studies on HBV, HCV, and HDV seroprevalence in Mongolia have been conducted in patients, donors, and children. There have been few studies performed on healthy individuals, especially adults aged ≥40 years, from both urban and rural areas. We believe that studying the prevalence of HBV, HCV, and HDV and hepatocellular carcinoma markers in this high-risk population with its associated risk factors will serve as valuable baseline data. These data can be used to evaluate the progress of current interventions and determine further improvements in public health measures to reduce and prevent viral hepatitis infections.
ACKNOWLEDGEMENTS
We would like to thank the participants for actively participating in our study and extend our thanks to all the contributing centers. We also would like to acknowledge invaluable support from the Department of Public Health and Division of Virology, Department of Infection and Immunity of Jichi Medical University, Japan.
Notes
CONFLICT OF INTEREST
The authors have no conflicts of interest associated with the material presented in this paper.
FUNDING
This study was provided by Jichi Medical University, Japan.
AUTHOR CONTRIBUTIONS
Conceptualization: Dambadarjaa D, Dayan A, Tsogzolbaatar EO. Data curation: Dayan A, Tsogzolbaatar EO, Oyunbileg NE, Shagdarsuren OE. Formal analysis: Khuyag SO, Mukhtar Y. Funding acquisition: Dambadarjaa D. Methodology: Dambadarjaa D, Nakamura Y, Takahashi M. Project administration: Dambadarjaa D, Takahashi M, Okamoto H. Visualization: Dambadarjaa D. Writing – original draft: Dambadarjaa D, Khuyag SO, Mukhtar Y, Shagdarsuren OE, Oyunbileg NE, Dayan A. Writing – review & editing: Dambadarjaa D, Nyam G, Nakamura Y, Takahashi M, Okamoto H, Tsogzolbaatar EO.