Cohort Study Protocol: A Cohort of Korean Atomic Bomb Survivors and Their Offspring
Article information
Abstract
In 1945, atomic bombs were dropped on Hiroshima and Nagasaki. Approximately 70 000 Koreans are estimated to have been exposed to radiation from atomic bombs at that time. After Korea’s Liberation Day, approximately 23 000 of these people returned to Korea. To investigate the long-term health and hereditary effects of atomic bomb exposure on the offspring, cohort studies have been conducted on atomic bomb survivors in Japan. This study is an ongoing cohort study to determine the health status of Korean atomic bomb survivors and investigate whether any health effects were inherited by their offspring. Atomic bomb survivors are defined by the Special Act On the Support for Korean Atomic Bomb Victims, and their offspring are identified by participating atomic bomb survivors. As of 2024, we plan to recruit 1500 atomic bomb survivors and their offspring, including 200 trios with more than 300 people. Questionnaires regarding socio-demographic factors, health behaviors, past medical history, laboratory tests, and pedigree information comprise the data collected to minimize survival bias. For the 200 trios, whole-genome analysis is planned to identify de novo mutations in atomic bomb survivors and to compare the prevalence of de novo mutations with trios in the general population. Active follow-up based on telephone surveys and passive follow-up with linkage to the Korean Red Cross, National Health Insurance Service, death registry, and Korea Central Cancer Registry data are ongoing. By combining pedigree information with the findings of trio-based whole-genome analysis, the results will elucidate the hereditary health effects of atomic bomb exposure.
INTRODUCTION
In August 1945, at the end of World War II, atomic bombs were dropped over Hiroshima and Nagasaki, Japan. Korea was a colony of Japan, and numerous Korean people were moved to Japan for conscription as workers in factories, mines, or soldiers, or to earn a living. Thus, Koreans who lived in Japan were affected by the atomic bomb. The number of Koreans affected by the atomic bombs was estimated to be 70 000, with 40 000 estimated deaths due to atomic bombs, and 23 000 atomic bomb survivors were estimated to have returned to Korea after liberation [1].
After World War II, several cohorts consisting of atomic bomb survivors (ABS), people who were exposed to atomic bombs in utero, and children of ABS in Japan were constructed. Based on cohort data, many research outcomes on the health and hereditary effects of radiation exposure due to atomic bombs have been reported. According to the Life Span Study [2], which consisted of ABS in Japan, atomic bomb radiation was significantly associated with increased solid cancer incidence [3], including salivary gland, esophageal, and stomach cancers [4]. Other evidence regarding radiation exposure unrelated to medical purposes and its health effects was obtained from studies after the Chernobyl disaster [5,6].
Studies on whether the effects of radiation exposure are inherited in offspring have shown inconsistent results. Research conducted on Japanese ABS offspring showed that radiation exposure to ABS was not associated with genetic mutations or health effects in the offspring [2,7], and a study of Chernobyl cleanup workers’ children found that transgenerational genetic effects were minimal [8]. In contrast, aberrant genome frequencies were increased both in Chernobyl cleanup workers and in their children born after the nuclear accident, suggesting the hereditary transmission of radiation-induced genomic instability [9].
In Korea, the ABS and their health and hereditary effects became public issues in the 1990s. Therefore, some studies about the health status of Korean ABS and their children have been conducted [10-17]. However, those studies focused on the health status of Korean ABS and their children using cross-sectional study designs; thus, the reasons for worse health status in ABS and their children than in the general population— that is, whether those differences were caused by atomic bomb exposure and its transgenerational effects, by health behaviors, or by socioeconomic differences compared with general population—could not be identified. Although ABS cohort studies in Japan investigated the health effects in ABS and their children, they compared the health status of ABS and their children with the general population and did not consider genetic effects or transgenerational effects of atomic bomb exposure. To date, there have been no studies on radiation exposure from atomic bombs and genetic effects between parents and children of atomic bomb survivors.
Study Objectives
The Korean Atomic Bomb Survivors Cohort (K-ABC) is a cohort study that aims to identify the association between atomic bomb radiation exposure and survivors’ health, as well as hereditary effects on their offspring’s health. The specific objectives of this study are as follows:
(1) To describe the health status of Korean ABS and their offspring and compare them with the non-exposed Korean population, adjusting for other confounders.
(2) To identify whether the radiation exposure related to the atomic bomb has transgenerational health effects.
(3) To examine whether the pattern of de novo mutations in the offspring of ABS is different from the non-exposed population.
(4) To examine whether different patterns of de novo mutations in offspring of ABS (identified in the third objective) have sufficient health effects to suggest transgenerational radiation-induced genetic changes.
METHODS
Cohort Study Participants
Definition of ABS and their offspring
In the K-ABC, the eligible population consists of Korean ABS and their offspring. A Korean ABS (G1) is defined as a person who meets the definition of “victim” in the Special Act On the Support for Korean Atomic Bomb Victims (“the special act” hereinafter). According to the special act, a “victim” includes (1) a person who was in Hiroshima or Nagasaki, Japan, at the time the atomic bomb was dropped; (2) a person who was within a 3.5 km radius of the hypocenter within 2 weeks after the atomic bombings; (3) a person exposed to radiation from an atomic bomb while being engaged in relief activities after the bombing; (4) a person who was exposed in utero at the time the atomic bomb was dropped; and (5) a person registered as a victim of the atomic bomb with the Korean Red Cross. The children of G1 are classified as “G2,” which stands for the second generation. The grandchildren of the G1 are classified as “G3,” which stands for the third generation.
Recruiting process
A total of 2093 G1, 2422 G2, and 2088 G1 individuals were registered with the Korea Atomic Bomb Casualty Association, Korea Atomic Bomb Casualty Offspring Association, and Korea Red Cross, respectively, in February 2021. In addition, postal mail, including research descriptions of the K-ABC and consent forms, has been sent to them. A total of 1668 (35.5%) ABS have returned the forms with signed informed consent.
To increase participation, the researchers have been visiting health examination sites for G1, provided by the Korean Red Cross, and have been promoting study participation. When G1 individuals agree to participate, they are asked to promote participation in this study with other family members, including siblings, children, and grandchildren. When G2 individuals participate in the study, they are asked to encourage their siblings and children to participate.
From 2020 to 2024, a total of 1500 G1, G2, and G3 individuals, including more than 200 trios, are planned to be recruited. This number of people can identify risk factors that increase the risk by 1.4-1.5 fold for disease with a prevalence of 3.9% (in the case of cancer), an alpha error of 5%, and a power of 80%.
Variables and Measurements
We have been collecting the following information from the K-ABC based on previous studies on ABS and their offspring [2,18,19].
Questionnaires
Trained interviewers have been administering the questionnaires, gathering responses on demographic information, atomic bomb exposure information, health status, and medical usage. Demographic information includes age, sex, residential area, family income, job, and education. The information regarding atomic bomb exposure includes the definition of “hibakusha” (which has the same definition as “victim” definitions 1 to 4 in the special act), the place of exposure, the distance from the hypocenter, shielding, symptoms after exposure, and long-term sequelae. Health status and medical use include subjective health status, diseases diagnosed by physicians, and medical radiation exposure history. For diseases diagnosed by physicians, this study includes congenital disorders, cardiovascular diseases, musculoskeletal disorders, endocrine disorders, malignant neoplasms, neuropsychiatric disorders, allergic diseases, eye disorders, ear diseases, genitourinary diseases, infertility, and pain due to unknown etiology. For medical radiation exposure history, the number of medical radiation exposures during the lifetime is asked for the following: X-ray (any type), dental panoramic X-ray, computed tomography, fluoroscopy, mammography, interventional radiography, radiation therapy (for cancer treatment), oral or intravenous administration of radioactive materials for diagnostic procedures, and administration of radioactive materials for treatment procedures. Health behavior information includes lifetime alcohol consumption, lifetime tobacco smoking, physical activity, sleep quality, and a brief assessment of dietary patterns. For female, reproductive factors are assessed, including menopausal status, age at menarche, number of pregnancies, age at first pregnancy, number of delivery experiences, age at first and last deliveries, duration of breastfeeding, age at first breastfeeding, number of artificial abortions, age at first artificial abortion, hysterectomy history, and salpingo-oophorectomy history.
Pedigree
As of 2021, 90% of G1 individuals who were registered as ABS with the Korean Red Cross had died, with a high proportion of deaths due to old age (the youngest G1 individuals were born on June 1, 1946 for Hiroshima or June 9, 1946 for Nagasaki). Most of the parents of G1 individuals were also exposed to the atomic bomb and most of the parents of G2 (thus G1) had died. Furthermore, those who suffered from severe atomic bomb after-effects immediately after exposure died early, and the atomic bomb survivors (G1) currently participating in the study are likely to have survival bias, which refers to the tendency for healthy people to survive longer. In addition, all of the parents of G1 died due to aging despite atomic bomb exposure. To reduce these biases, trained interviewers have been collecting pedigree information on first-degree relatives, which mean parents, siblings, and children, including birth year, vital status, year of death, cause of death (for dead people), past disease history, age at diagnosis, and atomic bomb exposure status. Although survival bias could not be excluded by pedigree information, we expect that this information will at least attenuate the problem.
Physical examination and laboratory tests
Trained investigators have been measuring height, weight, waist circumference, hip circumference, upper arm circumference, calf circumference, and blood pressure using standardized tools [20].
Free annual health screening examinations for ABS have been provided for G1 by Korean Red Cross at designated hospitals. Blood and urine samples have been collected during the health screening examinations for G1. For G2 and G3, we have been performing health screening examinations at a hospital where G1 individuals received health screening. For those who are unable to participate on weekdays, health screening was performed at Hanyang University Medical School and the Korea Atomic Bomb Victims Association with the presence of medical staff. During the health screening, blood and urine samples are collected.
Blood tests include random glucose, hemoglobin A1c, aspartate aminotransferase, alanine aminotransferase, gamma-glutamyl transferase, complete blood count, creatinine, blood urea nitrogen, total cholesterol, triglycerides, high-density lipoprotein cholesterol, and low-density lipoprotein cholesterol. The urine tests include pH, nitrate, specific gravity, protein, glucose, ketone, bilirubin, occult blood, urobilinogen, and leukocyte esterase. In addition, among the participants undergoing blood and urine tests, biological samples have been collected after obtaining consent for future genetic research from those who agreed to store and use serum, plasma, and urine for future research. Biomarkers such as interleukins, tumor necrosis factor-alpha, interferon-gamma, C-reactive protein, and erythrocyte sedimentation rate are planned to be measured.
To perform those tests, we collect 4 venous blood samples and one urine sample using 1 serum-separating tube (SST), 3 ethylenediaminetetraacetic acid (EDTA) tubes, and 1 conical tube. Considering those participants are very old, we do not obligate them to fast for more than 8 hours. When participants come to the hospital or research site, well-trained clinical laboratory technologists from Seegene Inc. (Seoul, Korea) or the nurses at the hospital perform blood sampling and urine sampling. We roll the EDTA tubes for 30 minutes immediately after the end of sampling to achieve thorough mixing with EDTA. The SST and conical tubes are set still after sampling. The SST is centrifuged for 10 minutes at 3000 rpm 30 minutes or more after sampling. After those procedures, those samples are refrigerated until they are transferred to the laboratory. If the samples are collected at hospitals, they are analyzed by the laboratory in that hospital and otherwise, the samples go to the nearest Seegene Inc. laboratory to be analyzed. The biomarker tests and conversion of the rest of samples into biospecimens are only done by Seegene Inc.
The SST is analyzed using Hitachi Labospect 008AS (Hitachi, Tokyo, Japan), the EDTA tubes are analyzed using XN-9000 (Sysmex, Kobe, Japan) and Automatic ESR Analyzer TEST1 (ALI Fax, Polverara, Italy), the conical tube is analyzed using a test strip and wavelength reflectance, and biomarkers are analyzed using enzyme-linked immunosorbent assays. The DNA extraction is done using the G-Dex IIb genomic DNA extraction kit for blood (Intron, Mevo Horon, Israel). To validate whether the hospital and Seegene Inc. show the same results, we give the blood and urine samples from the same people (at least 2) at the same time of sampling to those 2 laboratories every time when the hospital changes.
The remaining biospecimens, including plasma, serum, whole blood, and urine, are stored for future studies. Those biospecimens are processed by Seegene Inc. into 4 cryotubes, if possible, with 0.5 mL for each tube per biospecimen. The cryotubes are then sent to be stored at -70°C in a deep freezer.
Definition of trios
To identify whether de novo DNA mutations due to radiation exposure to atomic bombs in G1 have effects on the offspring of ABS, we have been collecting trios with parent-child relationships in G1, G2, and G3. Trios are classified into “case trios” and “control trios.”
For case trios, the definition of a “complete trio” is a trio including G1, G1’s spouse (spouse also can be G1), and at least 1 of their biological children. “Incomplete trios” are further divided into “reverse trios” and “linear trios.” A reverse trio comprises one G1 or G1’s spouses and 2 or more of their biological children. A linear trio is composed of 3 or more G2 individuals who have the same biological parents, of whom at least 1 belongs to G1 (Figure 1).
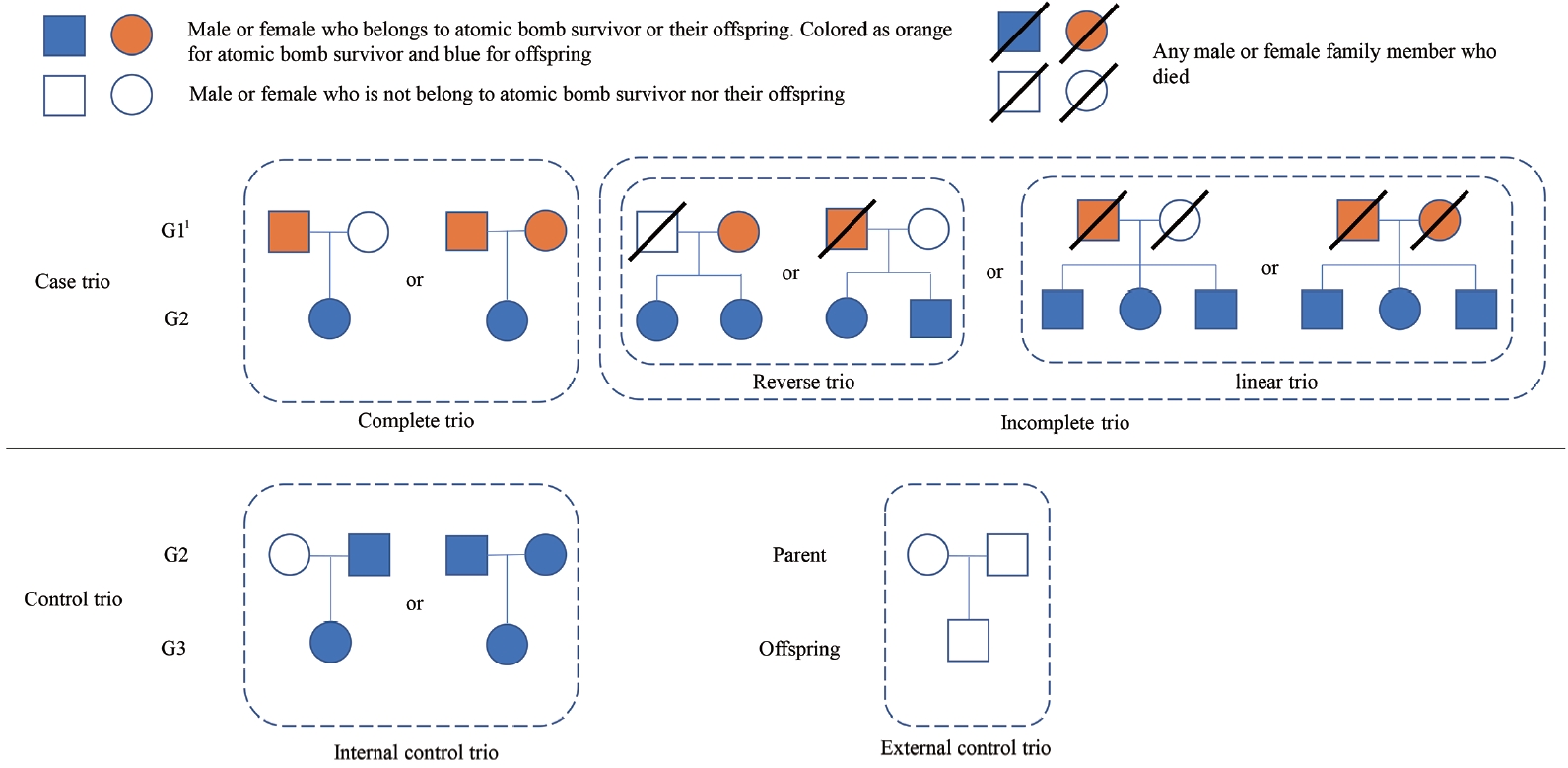
Pedigree chart showing structure of trio. 1G1, G2, and G3 represent the first generation, the second generation, and the third generation for each.
Control trios are categorized as “internal control trios” and “external control trios.” An internal control trio comprises parents of whom at least one is from G2, and their biological children belong to G3. An eternal control trio comprises parents and their biological children from the general population, such that none of their relatives within the third degree belong to the definition of G1, G2, or G3 (Figure 1).
Recruitment of trios
As there is no definite method to determine the number of trios needed to identify the associations of genetic variations, we used an estimation by Raffa and Thompson [21], which suggested 1500 parent-child pairs to find disease heritability. We also used experts’ advice, which suggested 1000 pairs for that purpose. As each trio can produce 2 or more parent-child pairs, we finally planned to recruit at least 200 case trios and 200 control trios. For the case trios and internal control trio, the study participants of the K-ABC are asked to promote the participation of family members if they want to identify their genome analysis results. External control trios are recruited from families of the general population through media sources.
Whole-genome sequencing
Whole-genome sequencing is planned to be performed for individuals who meet the criteria for trios. Next-generation sequencing (NGS) will be used to identify de novo mutations. A de novo mutation is a mutation that does not exist in parents, but newly develops in their children, and is produced by internal factors, such as DNA polymerase errors, or by external factors, such as radiation, ultraviolet light, or carcinogen exposure. De novo mutations will be measured by using the Genome Analysis Toolkit invented by the Broad Institute [22]. Subtypes of de novo mutations include single-nucleotide variations, small insertions/deletions, structural variations, copy number alterations, and chromothripsis. The presence of de novo mutations and genetic inheritance will be identified from the whole-genome information of trios.
Data Management
Whenever the survey is completed, the data are entered into a database (DB) program created for this study. We have been making telephone calls to the study participants to reconfirm any missing information found during DB entry.
There is a possibility of information bias in confirming the participants’ disease history through the survey. Especially, the mean age of the G1 participants is over 80 years, so participants sometimes find it difficult to remember the time of their disease diagnosis and the exact name of the diagnosis. To solve this problem, based on informed consent from the participants, data will be linked regularly to administrative data, including the Korea Red Cross, National Cancer Registry, and National Health Insurance Service. This data linkage will also be used for follow-up and for evaluating the reliability of the questionnaire (Supplemental Material 1). Furthermore, we tried to check the data validity. We have compared 88 participants’ cancer information between the questionnaire and the National Cancer Registry data in 2020. As a result, the kappa statistic for reliability was 0.61, suggesting a moderate agreement level (Supplemental Material 2).
Follow-up Survey
Both direct and indirect follow-ups are planned to identify newly developed diseases or deaths after the baseline survey. Direct follow-up will be conducted every 2 years after the first participation by mail or telephone. Indirect follow-up will be made by linking data to Statistics Korea mortality data, National Cancer Registry data, and National Health Insurance Service data through participants’ resident registration numbers.
Ethics Statement
The study protocol was approved by the Hanyang University Institutional Review Board (No. HYUIRB-202007-014-8). All participants have provided or will provide written informed consent after a full explanation of the purpose and process of the study. The collected data are anonymized after data linkage and stored in the DB.
RESULTS
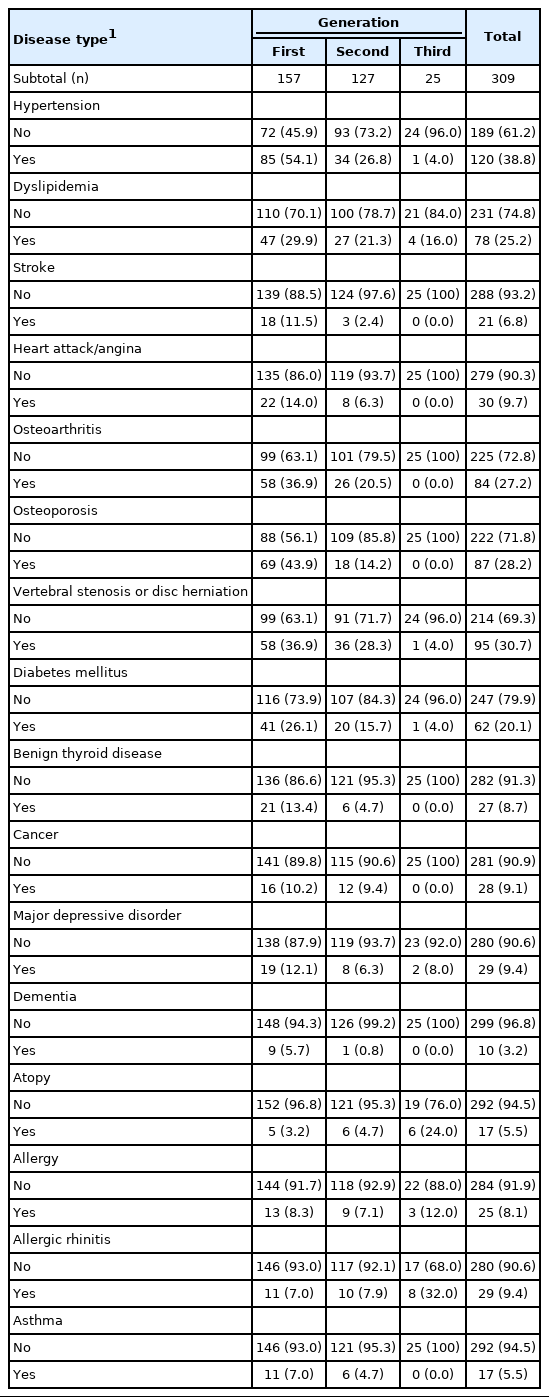
The preliminary results of the study participants recruited in 2020 are presented in this section. Table 1 shows the basic characteristics of 157 G1, 127 G2, and 25 G3 individuals. The mean and median ages of the G1 individuals were 82.4 and 81, respectively, and those of G2 were 61.4 and 63, respectively (Table 1).
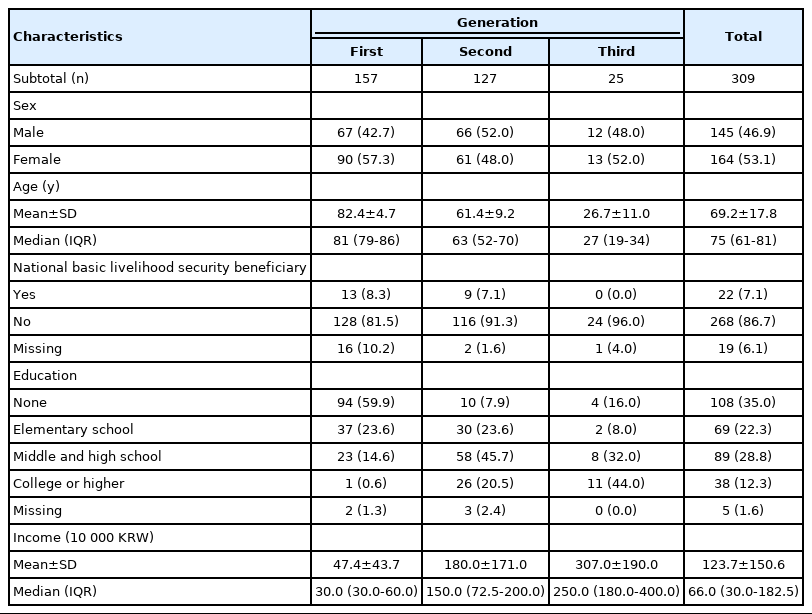
Basic characteristics of first-year participants in the Korean Atomic Bomb Survivors Cohort by generation
Table 2 lists the atomic bomb exposure data for G1. More than 50% of G1 individuals did not remember the definition of “hibakusha.” In combination with the old age of G1, secondary data linkage is necessary to supplement the information of G1. More than 90% of the G1 population was exposed in Hiroshima. In total, 35.7% of G1 individuals were located within 2 km from the hypocenter at the time of bombing (regarded as “inner proximal” as the definition of Life Span Cohort of Japanese survivors), 22.3% were located at 2.0-2.5 km from the hypocenter (regarded as “outer proximal” as the definition of Life Span Cohort of Japanese survivors), and 31.2% were located at 2.5-10.0 km from the hypocenter (regarded as “distal”) [23].

Survey information related to the atomic bomb exposure of the first generation among first-year participants
Table 3 shows the disease history of the participants by generation. The prevalence of most diseases was higher in G1, followed by G2 and G3. However, the prevalence of allergy-related diseases, such as atopy, allergy, and allergic rhinitis, was higher in G3 than that in G1 or G2. The most common disease diagnosed by a physician in G1 was hypertension (54.1%). The most common disease in G2 was vertebral stenosis or disc herniation (28.3%).
Information on 3068 first-degree family members, including 309 fathers, 308 mothers, 1280 brothers and sisters, and 946 children, was obtained from 309 participants (Table 4). More than 90% of fathers and mothers in G1 were G1 as well. More than 80% and 50% of the fathers and mothers of G2 had died, respectively; thus, including information on the dead parents of G1 and G2 would be necessary to reduce survival bias.
DISCUSSION
The strengths of this study are as follows. First, this is the first cohort study conducted on Korean ABS with biospecimens, and informed consent was obtained for linkage to various secondary data. Previous studies on Korean ABS were cross-sectional, with only questionnaire information. Thus, this study could contribute to biomarker studies of ABS with almost complete follow-up through linkage to secondary data using resident registration numbers. Second, this is the first whole-genome study based on the large number of ABS trios. Cohort studies conducted on ABS and their offspring in Japan showed no significant associations between the offspring and their cancer or chronic disease incidence [24-26], nor between the parents’ degree of exposure and the offspring’s disease [27]. However, concerns have been raised regarding the mechanisms of disease inheritance or non-inheritance due to radiation exposure from atomic bombs. Although a whole-genome study has been conducted on ABS and their offspring [7], the researchers used only 3 trios, with insufficient statistical power to determine the associations. Thus, results from the large number of trios (200 case and 200 control trios) could provide solid evidence for the hereditary effect of atomic bomb exposure. Third, although Japanese ABS cohorts were constructed shortly after atomic bomb exposure (the 1950s), these cohorts could not include people who had died right after atomic bomb exposure. This study collected pedigree information, including people who had already died. Thus, the heritability of atomic bomb exposure on death or disease development can be estimated.
Nonetheless, our study has several limitations. First, there could be selective survival bias. The source population of our study was people who lived at least 75 years after being exposed to atomic bombs, which means that they are healthier than the overall ABS population or the general population. Moreover, most G1 individuals identified by pedigree were dead. To adjust for this bias, we tried to obtain information about health status, exposure to atomic bombs, and cause of death of family members who did not or could not participate in our study through pedigree surveys. Second, some ABS hid their exposure to family members due to stigma toward ABS or people who had lived in Japan during the colonial period; therefore, some people are not aware that they are G2 or G3. Moreover, when we conducted the pilot survey, there was a testimony that an interviewee’s friend was exposed to the atomic bomb but did not receive Hibakusha Techo (a handbook) or register with the casualty association or Korea Red Cross. Those ABS and their offspring cannot be participants because they do not have the documents to prove, or do not know, their exposure status. Third, there can be information bias regarding the exposure of G1 to atomic bombs. Those in G1 who can participate in this study could have been very young when exposed to the atomic bomb, owing to which they would not be able to recall the situation vividly or answer using memories they heard from their parents, who were exposed to the atomic bomb but were already dead. To address this bias, we have been attempting to link our data with the Korean Red Cross data of Hibakusha Techo and the casualty association data on exposure to atomic bombs, generated via other investigations prior to our study. Fourth, most G1 participants are too old to recall information about their family members. To overcome these limitations, we have been including G1 and their children (G2) simultaneously to complete the data. Furthermore, we have been trying to link our data to National Cancer Registry and National Health Insurance Service to improve the reliability and validity of the data. However, the National Health Insurance Service data and the National Cancer Registry data can be linked from 2002 and 1999 onward, respectively; thus, health information before 2002 or 1999 could not be linked. Moreover, the National Health Insurance Service in Korea was first established for all Koreans in 1989, and there could have been people whose diseases were not diagnosed because of their limited access to medical care. Therefore, we need to use death information as a surrogate marker of overall disease status.
CONCLUSION
The results of our study may provide evidence that sheds light on whether exposure atomic bombs can affect the health status of offspring and the genetic mechanisms that could explain this evidence.
DATA AVAILABILITY
Data are only accessible to the researchers participating in this study.
SUPPLEMENTAL MATERIALS
Supplemental materials are available at https://doi.org/10.3961/jpmph.22.469.
Atomic Bomb Survivor and Their offspring Cohort Study design
Kappa statistics for cancer agreement between atomic bomb survivors and their offspring cohort in 2020 and Korea Central Cancer Registry data.
Notes
CONFLICT OF INTEREST
The authors have no conflicts of interest associated with the material presented in this paper.
FUNDING
This study was conducted with the support of the Ministry of Health and Welfare’s Academic R&D Service Project (grant No. 11-1352000-003268-01).
AUTHOR CONTRIBUTIONS
Conceptualization: Park B, Moon SG, Jeong A. Data curation: Moon SG, Jeong A, Han Y, Park B. Formal analysis: Moon SG. Fungind acquisition: Park B. Methodology: Park B, Nam JW. Project administration: Moon SG, Jeong A, Han Y. Writing – original draft: Moon SG, Jeong A, Park B, Han Y, Nam JW, Kim MK, Kim I, Kim YM. Writing – review & editing: Moon SG, Jeong A, Park B, Han Y, Nam JW, Kim MK, Kim I, Kim YM.
ACKNOWLEDGEMENTS
We thank all the participants and researchers, especially Yunja Han, Eun Hee Yu, the Korea Atomic Bomb Casualty Association, and the Korea Atomic Bomb Casualty Offspring Association.