Cancer Incidence in Korean Vietnam Veterans During 1992-2003: The Korean Veterans Health Study
Article information
Abstract
Objectives
The aim of this study was to investigate the association between Vietnam experience including exposure to military herbicides and cancer incidence in Korean Vietnam War veterans.
Methods
The cancer cases of 185 265 Vietnam veterans from January 1, 1992 to December 31, 2003 were confirmed from the Korea National Cancer Incidence Database. The age-adjusted incidence and standardized incidence ratios (SIRs) were calculated using the male population during 1992 to 2003 as a standard population.
Results
The age-adjusted overall cancer incidence per 100 000 person-years was 455.3 in Vietnam veterans. The overall cancer incidence was slightly yet significantly lower in veterans (SIR, 0.97; 95% confidence interval, 0.95 to 0.99) than in the general population. The overall cancer incidence in enlisted soldiers was not lower (SIR, 1.00), whereas that in officers was significantly lower (SIR, 0.87) than in the general population. The incidences of prostate cancer and T-cell lymphoma in all veterans, and lung cancer and bladder cancer in enlisted soldiers, and colon cancer and kidney cancer in non-commissioned officers, and colon cancer, kidney cancer, and prostate cancer in officers, were higher than in the general population. The SIR for overall cancer among Vietnam veterans rose from 0.92 for 1992-1997 to 0.99 for 1998-2003.
Conclusions
The overall cancer incidence in Vietnam veterans was not higher than in the general male population. Vietnam veterans and military rank subcohorts experienced a higher incidence of several cancers, including prostate cancer, T-cell lymphoma, lung cancer, bladder cancer, kidney cancer, and colon cancer than the general population. The SIR for overall cancer increased over time in Vietnam veterans.
INTRODUCTION
During the Vietnam War, between 1961 and 1971, the US and allied forces sprayed herbicides for military purposes [1]. The herbicides, containing phenoxy herbicides as a major ingredient, were coded as Agent Green, Orange, Pink, Purple, and White. During the manufacturing process, 2,4,5-T, an ingredient of phenoxy herbicides, was contaminated by 2,3,7,8-tetrachlorodibenzo-p-dioxin (TCDD), the most toxic dioxin congener. The estimated TCDD levels in these military herbicides were 13-66 ppm on average [2,3]. In the US, the manufacturing standards for domestic use of 2,4,5-T in 1974 required that TCDD levels be less than 0.05 ppm. The TCDD being used during the Vietnam War surpassed 1000 times the permitted density limits [4]. It is estimated that the total amount of dioxin sprayed during the Vietnam War ranged from the minimum of 366 kg to the maximum of 1000 kg [2].
From the initial deployment of a support unit in 1964 until its complete withdrawal in 1973, the Korean military sent 320 000 military personnel [5]. It is presumed that many Korean Vietnam veterans were exposed to toxic herbicides, including TCDD. TCDD is being classified by the International Agency for Research on Cancer (IARC) as a group 1 carcinogen that is carcinogenic to humans [6]. Likewise, the US National Toxicology Program listed it as a known human carcinogen [7].
While the major news media and Vietnam veterans in Korea have expressed their concerns about veterans' health problems, no empirical study has been published in a scientific journal exploring whether the cancer incidence of Korean Vietnam veterans, who encountered Agent Orange, an unfamiliar climate, food and water, and infectious diseases, as well as war-related stress in Vietnam, is different from that of the Korean male population. Corresponding studies on Vietnam veterans in America and Australia [8-10] have limitations in exploring infrequent and rare cancers due to their relatively small sample sizes [8,9]. Further, due to ethnic differences, the direct comparison between Korean Vietnam veterans and Western counterparts may present some challenges [10,11].
The purpose of this study was thus to identify Korean Vietnam veterans' incidence of cancer of all sites combined as well as specific sites, to compare them with the general Korean population of the same age group, and to investigate whether there were differences in cancer incidence between Vietnam veterans and the general population in Korea.
METHODS
Study Subjects
With the cooperation of the Ministry of Defense and then the Ministry of Government Administration and Home Affairs, the author and colleagues identified 187 897 veterans during 1999 to 2000 and confirmed their official residential status as of June 2004 [12]. The Korean Veterans Health Study included these 187 987 veterans, and it was established to evaluate primarily the association between Vietnam experience and Agent Orange exposure, and the morbidities and mortality from various diseases. Most of the information on individuals deceased before January 1, 1992 was deleted from the Korean Resident Registration Database. Therefore, after excluding 2137 individuals who were deceased or had emigrated before 1992, and 495 people who were reported to have cancer before December 31, 1991, in the end, 185 265 veterans were selected as the study cohort in order to trace cancer incidence starting from January 1, 1992. The research project was approved by the Institutional Bioethics Committee of Kwandong University.
Cancer Incidence Follow-up
The data from the National Cancer Incidence Database (NCID) from 1988 to 2003 enabled us to confirm the veterans' cancer incidence and cancer site. December 31, 2003 was the last date of the cancer incidence follow-up. The cancer statistics of the NCID were listed in official reports of the IARC [13].
Cancer Classification
The International Classification of Diseases, tenth revision (ICD-10) code from the NCID was utilized to categorize cancer by cancer site. The classification followed the categorization by the IARC report [13]. Non-Hodgkin lymphoma and leukemia with 10 or more incidents were segmented by 4-digit categorization of the ICD-10 code, and were included in the analysis.
Age Standardization and Analysis
Cancer incidence of veterans was calculated from follow-up person-years and cancer cases. Since the range of veterans' ages during 1992 to 2003 was 36 to 81, the population estimate of males aged 36 to 81 years from the National Statistical Office of Korea was used. The standard population for direct age standardization was all males aged 36 to 81 years during 1992 to 2003 (216 906 479 males), while that for indirect standardization was Korean males with the same age per calendar year during 1992 to 2003. The cancer incidence in the general population (population rate) was calculated with a total of Korean males aged 36 to 81 years during 1992 to 2003 (216 906 479 persons), along with the number of cancer cases.
When a case of cancer was identified by December 31, 2003, the date of diagnosis was considered the end of the follow-up period. When an individual's residential record was cancelled (unknown residence), or a veteran emigrated to another country, the date the residential status changed was the date of loss to follow-up. The number of person-years by 1-year age group by each calendar year was calculated.
The age-adjusted cancer incidence rate was calculated by applying 5-year age-specific rates (years of age: 36-39, 40-44, 45-49, 50-54, 55-59, 60-64, 65-69, 70-74, 75-79, and 80-81) to the standard population. The expected number of cancer cases was calculated by applying 1-year age-specific rates by calendar year to the follow-up person-years by calendar year in veterans, and standardized incidence ratios (SIRs) were calculated.
When the SIR was bigger than 1, the cancer incidence of veterans was higher than that of the general population. When the SIR was smaller than 1, the cancer incidence of veterans was lower than that of the general population. The 95% confidence interval (CI) and p-value were calculated by the Poisson asymptotic method [14]. All of the statistical analyses were performed using SAS version 9.2 (SAS Inc., Cary, NC, USA).
For major cancers with 100 or more incidents, additional analysis was performed, stratifying by military rank (military rank subcohorts: the enlisted, non-commissioned officers, and officers) or follow-up period (the first half [1992 to 1997] and the latter half [1998 to 2003]).
RESULTS
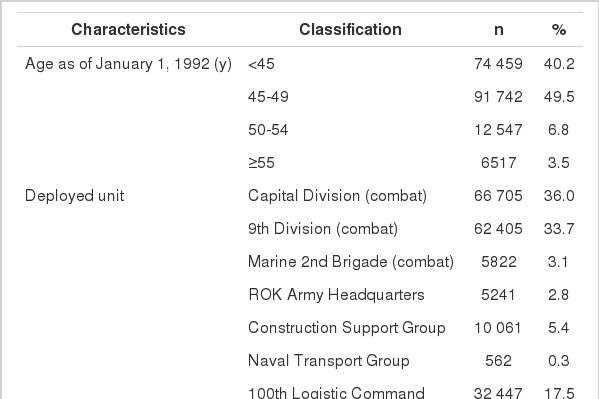
An average age of Korean veterans was 46.3 years (±3.5 years) as of January 1, 1992. The Capital Division and the 9th Division made up the vast majority of the Korean Army. Enlisted personnel, with about 145 000 troops, made up the most common rank, while field officers or generals comprised 2724 personnel. For the year of the initial deployment to Vietnam, the period of 1969 to 1970 had the largest number of personnel, with 56 000 troops (Table 1).
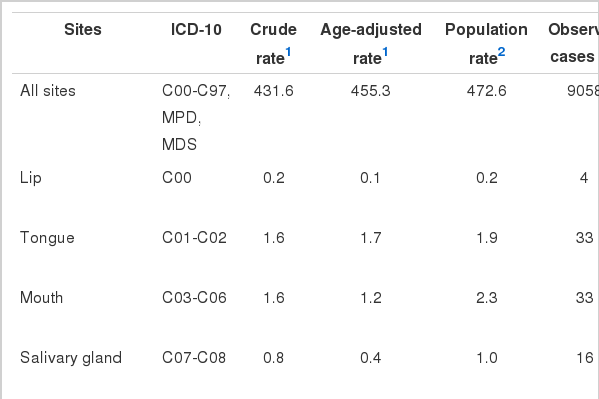
The total follow-up person-years was 2 098 602, and 9058 veterans were diagnosed with cancer up to December 31, 2003, the end of the follow-up period, in 185 265 veterans. The crude incidence rate of all sites of cancer (hereafter, "all cancer") in the veterans was 431.6 per 100 000 person-years and the age-adjusted rate was 455.3 per 100 000 person-years. For the period of 1992 to 2003, the all-cancer incidence rate in the general population aged 36 to 81 years old was 472.6 per 100 000 (Table 2). The most common cancers in the general population were stomach cancer, liver cancer, and lung cancer in sequence, while those in veterans were stomach cancer, lung cancer, and liver cancer. The SIR for all cancer in the Vietnam veterans was significantly lower than that of the general population (SIR, 0.97; 95% CI, 0.95 to 0.99; p=0.04). The veterans' incidence of prostate cancer (p=0.03) was significantly higher and the rate of T-cell lymphoma (p=0.07) was marginally higher than that of the general population. In the meantime, the incidences of pharyngeal cancer (p=0.04), esophagus cancer (p<0.001), and gastric cancer (p<0.001) in veterans were significantly lower than in the general population (Table 2).
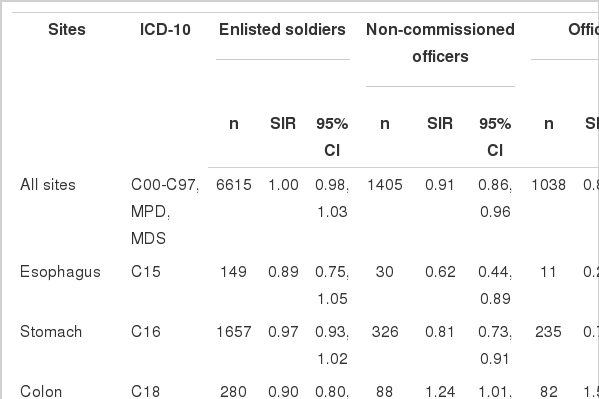
The SIR for all cancer in the enlisted veterans was not different from the general population (SIR, 1.00; 95% CI, 0.98 to 1.03) (Table 3). The incidence of lung cancer (p=0.002) was higher than in the general population, and the rate of bladder cancer (p=0.064) was marginally higher than in the general population. The enlisted did not have significantly lower SIRs for any cancers than in the general population. In contrast to the enlisted, the noncommissioned officers (SIR, 0.91; p<0.001) and officers (SIR, 0.87; p<0.001) had a significantly lower cancer incidence than the general population. Among the non-commissioned officers, the colon cancer incidence was higher (p=0.04) and kidney cancer incidence was marginally higher (p=0.096) than in the general population, while the cancer incidences of the esophagus (p=0.01), stomach (p<0.001), and lung (p=0.047) were significantly lower than in the general population. Among the officers, the colon cancer (p<0.001) and prostate cancer (p<0.001) incidences were higher than in the general population, and the kidney cancer (p=0.097) incidence was marginally higher than in the general population, while the incidences of esophagus, (p<0.001), stomach (p<0.001), liver (p<0.001), larynx (p=0.02), and lung cancer (p<0.001) were significantly lower than in the general population (Table 3).
The cancer incidence of the veterans from 1992 to 1997 was significantly lower (p<0.001) than in the general population, while the rate from 1998 to 2003 did not differ from that of the general population. For the 1992 to 1997 period, no cancer in veterans had a significantly higher incidence than in the general population. However, during the period of 1998 to 2003, the prostate cancer incidence of the veterans was marginally higher (p=0.05) than in the general population (Table 4).
DISCUSSION
This study showed that the incidence of all cancer in Korean Vietnam veterans was modestly lower than in the general population. Past research has demonstrated that all-cancer incidences in US Ranch Hand veterans (SIR, 1.08) and Australian veterans (SIR, 1.15) were not lower than in the general population [8,10], whereas the incidence in occupationally TCDD-exposed workers was lower than in the general population [15,16]. In mortality studies, Vietnam veterans have usually been found to have a lower death rate than the general population [5,8,17-19]. A "healthy soldier effect" can explain why the all-cancer incidence in the veterans was lower than in the general population. The effect is a type of healthy worker effect, a phenomenon well-documented in the occupational epidemiology field. Although Korea has a universal conscription system and Korean men have an obligation to perform military service, the status of full-time active duty of the enlisted is determined by physical examination. The troops sent to the Vietnam War were rigorously selected among volunteer servicemen in terms of physical fitness, educational status, family background, and other criteria. For the Capital Division, the enlisted who were selected were only 20% to 30% of the volunteers [20]. Therefore, the selected should be more fit and stronger than the general population, or even non-Vietnam peers with military experience. However, although the Vietnam veterans were healthier than the general population, the difference in the cancer incidence between the Vietnam veterans and general population was small. For the enlisted, the incidence of all cancer was the same as that of the general population.
In the present study, compared to the general population, the veterans had a significantly elevated incidence of prostate cancer, and a marginally significantly elevated incidence of T-cell lymphoma (C84), a type of non-Hodgkin lymphoma. In the enlisted, the lung cancer incidence was significantly higher and bladder cancer incidence was marginally higher than in the general population. In the non-commissioned officers, the colon cancer incidence was significantly higher and the kidney cancer incidence was marginally higher than in the general population. In the officers, the risks of colon cancer and prostate cancer were significantly higher and the risk of kidney cancer was marginally higher than in the general population. Overall, the Korean Vietnam veterans and military rank subcohorts had higher incidences of several cancers, including prostate cancer, T-cell lymphoma, lung cancer, bladder cancer, kidney cancer, and colon cancer than those of the general population. Prostate cancer, non-Hodgkin lymphoma, and lung cancer have been shown to have relationships with TCDD or military herbicides in a previous literature review [1]. Several studies on the IARC phenoxy herbicide cohort, National Institute for Occupational Safety and Health cohort, and Ranch hand veterans, who were occupationally exposed to high levels of TCDD, have reported that the mortality from urinary system cancer, including bladder cancer and kidney cancer, was higher in TCDD-exposed workers and veterans than in control groups; not all of the elevated mortality findings were significant, though [8,21,22]. The incidence of colon cancer among Australian Veterans was a little higher than that of the general population [10], while US military studies and some occupational cohort studies did not report significant relationships [8,21,22].
Soft tissue sarcoma (for the current study, connective and soft tissue cancer: D47+D49), chronic lymphocytic leukemia, Hodgkin lymphoma, laryngeal cancer, and multiple myeloma, all of which have previously been shown to have relationships with military herbicides/TCDD-related chemicals [1], did not have a significantly elevated incidence in Vietnam veterans in the present study. However, considering healthy soldier effects, the fact that the veterans did not have a high cancer incidence compared to the general population does not necessarily mean that Vietnam experience, including Agent Orange exposure during the Vietnam War, are not related to the incidence of those cancers. In order to investigate the effects of Agent Orange exposure on cancers while minimizing healthy soldier effects, the incidence of cancers in Vietnam veterans should be compared to more comparable internal controls such as Vietnam veterans who were not exposed to Agent Orange rather than the general population [5]. In the meantime, when the cohort of the Korean Veterans Health Study was established, the author and colleagues spoke with the then Ministry of Government Administration and Home Affairs about selecting Korean males who had been full-time soldiers on active military duty during the Vietnam era as controls. However, access to data on such controls did not materialize, partly due to privacy concerns.
While TCDD is a proven carcinogen in animal experiments, some researchers have been questioning whether TCDD is a multiorgan carcinogen to humans [23]. Although prostate cancer was shown to have a higher incidence in Korean Vietnam veterans than in the general population, and has been suggested to have a strong connection with Agent Orange or TCDD in US Vietnam veterans, the mechanism of how TCDD affects the prostate has not been clearly identified. It is thought that aryl hydrocarbon receptor (AhR), a protein known to mediate the toxicity of TCDD, could play a role in the carcinogenesis of prostate cancer. However, the ostensibly contradictory mechanisms by which AhR promotes prostate cancer and prohibits prostate carcinogenesis have been presented in animal experiments [24]. In US veterans, some studies have confirmed that the high TCDD exposure group had an increased incidence of prostate cancer [8], while other studies have shown that the group with high exposure to TCDD had a decrease in prostate hyperplasia, a high risk factor for prostate cancer [25].
In the present study, officers with ages of lower than 45, 45-49, 50-54, 55-59, and 60 or more years as of 1992 had SIRs of 4.6, 2.2, 3.2, 2.6, and 1.4 for prostate cancer, and generally, the younger the veterans were, the higher the SIR of prostate cancer was compared to the general population. This result concurs with a previous study of US veterans [26]. The current study reported a high SIR of prostate cancer for officers only. Since the enlisted and non-commissioned officers were younger by 6 and 3 years than the officers, respectively, extending the follow-up would be needed to investigate whether the prostate cancer incidence will increase in the future.
The current study found that the all-cancer incidence in the veterans during 1992 to 1997 was lower than in the general population, yet the incidence during 1998 to 2003 was close to the incidence of the general population. Many occupational studies, including research on veterans, have found lower mortality or cancer incidence in TCDD-exposed subjects during an initial stage of follow-up than in the general population, and then incremental rates of change thereafter [18,27]. This could be explained by the gradual disappearance of advantages in the selection process of employment-in other words, a decrease in the healthy worker effect over time-on the other hand, it could be a consequence of accumulated harmful effects of past exposure [27]. If the healthy worker effect were to simply diminish with time, the cancer incidence in Vietnam veterans would eventually become similar to that of the general population. If the hazardous effects of past exposure were to finally take a toll on veterans, the cancer incidence in veterans could be higher than in the general population as time passes. The SIR of all cancer in the enlisted during 1998 to 2003 was 1.02 (95% CI, 0.99 to 1.06; p=0.12). This was not a statistically significant difference from the general population, but it was slightly higher. Thus, it is recommended that further follow-up be performed to explore whether the cancer incidence in Vietnam veterans becomes higher than in the general population in the future.
The current study demonstrated that a rise in military rank had an inverse relationship with the incidence of all cancer, and officers had the lowest all cancer incidence among Vietnam veterans. Furthermore, the SIRs of some specific cancers were different among military rank subcohorts. The fact that socioeconomic status (SES) and SES-related demographic characteristics, lifestyle, medical utilization characteristics, and so on can affect the incidence, mortality, and survival of various cancers has been well established [11,28]. In the 1960s and 1970s, Korean officers, compared to enlisted men and noncommissioned officers, were an elite group with a high SES who had graduated from the military academy; therefore, a high SES-related characteristics and lifestyle may explain the observed difference in SIRs of some cancers compared to veterans with other military ranks. The officers had lower serum TCDD concentrations than the enlisted in the Air Force Health Study [29]. Thus, we cannot rule out the possibility that Korean veterans may have different levels of exposure to Agent Orange by military rank, although there has not been conclusive evidence that Korean officers were less exposed to TCDD-contaminated military herbicides than the enlisted.
There were distinctive differences in the incidence of some cancers according to the veterans' military rank. With increasing military rank, the incidence of cancers of the esophagus, stomach, and lung clearly decreased. Additionally, the officers had a significantly lower incidence of liver cancer and laryngeal cancer than the general population. Meanwhile, the incidence of colon cancer increased with increasing military rank, and the officers had a significantly higher incidence of prostate cancer than the general population. These results are in accord with previous research that shows the risks of gastric cancer [30,31], esophageal cancer [31,32], lung cancer [28,33], liver cancer [34], and laryngeal cancer [35] have inverse relationships with SES, while the risk of colon cancer [36] and prostate cancer [28] have positive relationships with SES. This agreement with previous studies as well as the low all-cancer incidence in the officers, suggest that military rank reflects SES in Vietnam veterans, at least partially.
One of the strengths of this study lies in the opportunity to identify the risk of rare cancers due to the large number of subjects. As a cancer incidence study based on a nationwide cancer registry, the diagnosis of cancer was more accurate, and cancers with a low lethality were detected more frequently than in mortality studies [37]. At the same time, the current study is not without limitations. First, follow-up on the study cohort began in January 1992, when at least 19 years or at most 27 years had passed after the veterans had returned from Vietnam. If veterans with severe consequences due to Vietnam service or Agent Orange exposure had cancer or were deceased before January 1, 1992, this study may have underestimated the cancer incidence in Korean Vietnam veterans. However, considering the fact that it usually takes 10 or more years to develop cancer after exposure to toxic chemicals, the above challenge may present fewer problems than in a mortality study [5]. Second, even though the quality of the Korea NCID has substantially improved in recent years, it had some limitations in the early and mid-1990s [38]. Since this presents the same challenges for both veterans and the general population, the author does not consider it to have caused a substantial bias in the SIR estimation. Third, the fact that this study controlled for only age and calendar year of death and could not adjust for important risk factors for mortality such as smoking, drinking, and obesity, was another challenge. However, it is practically impossible to control these variables, when comparing the cancer incidence of subjects with that of the general population. Thus, the author does not think this is a unique challenge only applicable to the current study [27]. Finally, the current study's follow-up period was 12 years, which was shorter than some of the existing research on veterans, workers, or residents occupationally or environmentally exposed to toxic chemicals [10,15,37]. Nevertheless, the substantial follow-up in person-years due to the large sample size was strong enough to compensate for the slightly shorter follow-up period.
In conclusion, the cancer incidence in Vietnam veterans was modestly yet significantly lower than in the general population. This could be explained by the "healthy soldier effect". In all of the Vietnam veterans, the incidence of prostate cancer and T-cell lymphoma (C84) was higher than in the general population, while the enlisted soldiers had a higher incidence of lung cancer and bladder cancer than the general population. Whereas the noncommissioned officers and officers had a lower all-cancer incidence than the general population, the enlisted had a similar all-cancer incidence to the general population. The incidence of cancers of the esophagus, stomach, liver, larynx, lung, and prostate differed according to military rank in the Vietnam veterans. The all-cancer incidence in the Vietnam veterans was lower than in the general population during 1992 to 1997, whereas the incidence was similar to that in the general population during 1998 to 2003. Ongoing follow-up is needed to investigate whether the incidence of all cancer as well as specific cancers has an increasing trend relative to the general population.
ACKNOWLEDGEMENTS
This study was supported by a research grant of the Korean Ministry of Patriots and Veterans Affairs, 1999, 2003, and 2009. The author greatly appreciates Professor H. Ohrr for his advice and support on preparing this paper. The author also truly thanks the staffs of the National Cancer Center of Korea for the NCID.
Notes
The author has no conflicts of interest with the material presented in this paper.