Articles
- Page Path
- HOME > J Prev Med Public Health > Volume 56(5); 2023 > Article
-
Original Article
Clinical Features and Risk Factors of Post-COVID-19 Condition in Korea -
Myungwon Jang1
 , Dongkwon Choi2
, Dongkwon Choi2 , Jonghyuk Choi2,3
, Jonghyuk Choi2,3 , Ho-Jang Kwon2,3
, Ho-Jang Kwon2,3
-
Journal of Preventive Medicine and Public Health 2023;56(5):431-439.
DOI: https://doi.org/10.3961/jpmph.23.124
Published online: September 8, 2023
- 1,224 Views
- 78 Download
1Epidemic Intelligence Officer, Dangjin City Public Health Center, Dangjin, Korea
2Chungnam Center for Infectious Diseases Control and Prevention, Hongseong, Korea
3Department of Preventive Medicine, Dankook University College of Medicine, Cheonan, Korea
- Corresponding author: Ho-Jang Kwon, Department of Preventive Medicine, Dankook University College of Medicine, 119 Dandae-ro, Dongnam-gu, Cheonan 31116, Korea, E-mail: hojangkwon@gmail.com
Copyright © 2023 The Korean Society for Preventive Medicine
This is an Open Access article distributed under the terms of the Creative Commons Attribution Non-Commercial License (https://creativecommons.org/licenses/by-nc/4.0/) which permits unrestricted non-commercial use, distribution, and reproduction in any medium, provided the original work is properly cited.
ABSTRACT
-
Objectives
- Numerous studies have explored the causes and spread of outbreaks, yet there is a lack of research on post-coronavirus disease 2019 condition (PCC) in Korea. The goal of this study was to identify the various types of PCC and associated factors in discharged patients and to provide directions for the ongoing health management of confirmed patients.
-
Methods
- A telephone survey was conducted among 680 coronavirus disease 2019 (COVID-19) patients diagnosed between July 7, 2021 and August 26, 2021, in Dangjin, Chungnam, Korea. A descriptive analysis of characteristics, univariate analysis, and regression were performed using data from basic epidemiological surveys conducted at the time of diagnosis and post-discharge questionnaires.
-
Results
- Of the 585 patients who responded, 159 (27.2%) developed PCC. Of the 211 patients with no initial symptoms, 27 (12.8%) developed PCC, versus 132 (35.3%) of the 374 patients with initial symptoms. Among the initial symptoms, fever or chills, cough or sputum, loss of smell, and sore throat were associated with PCC. Compared to patients with less than 10 days of hospitalization, those with a hospitalization period of 21 days to 30 days (odds ratio [OR], 2.3; 95% confidence interval [CI], 1.0 to 5.2) and 31 days or more (OR, 5.8; 95% CI, 1.9 to 18.1) had a higher risk of PCC.
-
Conclusions
- More than a quarter of COVID-19 patients, including those who had no initial symptoms, experienced PCC in Korea. People with the initial symptoms of fever, chills, and respiratory symptoms and those who had prolonged hospital stays had a high risk of PCC.
- Coronavirus disease 2019 (COVID-19), first reported in Wuhan, China, in December 2019, is transmitted by the severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) pathogen through droplets, surface contact, and airborne transmission in limited circumstances. Originating in China, it quickly spread globally, leading to its declaration as a pandemic on March 11, 2020. Korea reported its first domestic case of COVID-19 on January 20, 2020. By November 10, 2022, over 25 900 000 individuals had been infected with SARS-CoV-2.
- According to the COVID-19 Mutant Virus Guidelines from the Korea Disease Control and Prevention Agency (KDCA), five major variants of concern have been identified to date: Alpha (α), Beta (β), Gamma (γ), Delta (δ), and Omicron (ΰ) [1]. Each variant exhibits different levels of contagiousness and severity. As of March 2021, the Alpha variant, which was the first variant of concern in Korea, was reported to have the highest severity among the Alpha, Beta, and Gamma variants [2]. The Omicron variant, which triggered the fifth wave of infections in Korea in the first half of 2022, was found to be 2–3 times more infectious but less severe than the Delta variant. This led to a significant surge in domestic cases in February 2022 and March of 2022.
- The clinical manifestations of COVID-19 range from asymptomatic to severe, with primary symptoms including fever (above 37.5°C), cough, shortness of breath, chills, muscle pain, and loss of smell. Additional symptoms may encompass fatigue, loss of taste, sputum production, and gastrointestinal issues. A study on the clinical characteristics of COVID-19 revealed a median incubation period of 5.1 days. When exposed to an infected family member, fever and respiratory symptoms typically manifest within 3–7 days of exposure. However, symptoms such as a sore throat and muscle pain are comparatively rare [3].
- The clinical symptoms of COVID-19 can manifest differently based on factors such as vital signs, sex, and age, and this variability has been the focus of numerous studies [4]. Additionally, with an increasing number of patients reporting persistent symptoms or the emergence of new symptoms after a certain period, the World Health Organization (WHO) formally defined the post-coronavirus disease 2019 condition (PCC) on October 21, 2021.
- The WHO defines PCC as symptoms that typically appear 3 months following the onset of COVID-19 and persist for at least 2 months, which cannot be attributed to other diagnoses [5]. While most patients fully recover, approximately 10–20% continue to experience various symptoms during the mid-term to long-term course of the disease, even after the initial symptoms of infection have subsided. This phenomenon occurs regardless of the severity of the initial symptoms or hospitalization, and it is important to note that these patients are not infectious, even if PCC symptoms persist [6]. The Centers for Disease Control and Prevention (CDC) in the United States characterizes PCC as symptoms that persist for 4 or more weeks after the initial infection, encompassing new, recurring, or ongoing health issues [7].
- A prospective study involving 130 patients in Korea identified changes in clinical features on chest CT images, dyspnea, and loss of smell and taste as PCC [8]. A separate study, which compared COVID-19 and influenza using data from the Health Insurance Review and Assessment Service, found that 19.1% of patients with COVID-19 and 28.5% of patients with influenza sought medical care due to complications [9]. Furthermore, Kim et al. [10] reported that advanced age, female sex, and symptom severity were risk factors for PCC with persistent neurological symptoms.
- Menges et al. [11] found a correlation between the severity of initial symptoms and comorbidities with PCC. Similarly, Zhang et al. [12] discovered an association between the severity of COVID-19 and PCC. Another study revealed an association between the number of symptoms in the acute phase of COVID-19 and PCC [13]. However, previous studies were somewhat limited, as they were either hospital-based or did not compare the effects on PCC in relation to the manifestation and type of initial symptoms. The aim of this study was to explore the distribution of PCC and to ascertain how factors such as initial symptoms, underlying diseases, and the length of hospitalization might impact the incidence of PCC.
INTRODUCTION
- The US CDC defined PCC as a range of symptoms that can persist for at least four weeks, or even several months, following infection. We conducted a case analysis based on this definition at the time of our investigation. We chose to use the CDC’s criteria over the WHO’s because the latter’s definition is more stringent, which would have reduced our study sample size; instead, our aim was to include a wide range of subjects. The case analysis used a questionnaire developed by the Dangjin City Public Health Center, and a survey was conducted to obtain basic data for follow-up management after discharge.
- The survey was carried out at the Dangjin City Public Health Center from July 7, 2020, to September 25, 2021. It involved 750 patients who had been confirmed positive for COVID-19 through laboratory tests (reverse transcriptase polymerase chain reaction). To align with the US CDC definition, we included 680 confirmed cases diagnosed by August 26, 2021—4 weeks prior to the conclusion of the study—as our subjects. Data collection was conducted via basic epidemiological surveys, in accordance with local government guidelines for COVID-19, and through telephone questionnaires. Information such as sex, age, ethnicity, underlying health conditions, and, and initial symptoms at diagnosis were obtained from the basic epidemiological survey, and PCC information was obtained from a 1:1 telephone questionnaire.
- PCC was categorized into 7 body systems corresponding to those used in the review of systems: systemic, skin, head/neck/ear/nose and throat, musculoskeletal, neuropsychiatric, respiratory or circulatory, and digestive symptoms. Descriptive statistics were presented for the types and distribution of PCC, and associations between the general characteristics and PCC were analyzed through univariate analysis using the chi-square test. The effects of various risk factors on PCC were analyzed through multiple logistic regression adjusting for various factors, where p-value was the probability of PCC.
- The level of significance for all analyses was set at 0.05, and all analyses were conducted using SPSS version 26.0 (IBM Corp., Armonk, NY, USA).
- Ethics Statement
- This research was conducted using existing data or documents related to our research subjects, and it was carried out with the approval of an exemption from the Institutional Review Board of Dankook University (IRB No. DKU 2022-12-006).
METHODS
- A total of 585 confirmed cases were finally included, with the exclusion of 95 patients who refused to take part in the survey, changed their phone number, did not answer calls, or died before the survey took place. The response rate of the survey was 86.0% (585 out of 680).
- Out of a total of 585 subjects, 310 (53.0%) were male and 275 (47.0%) were female. Among them, 77 (24.8%) male and 82 (29.8%) female had PCC. There was no statistically significant difference in PCC by sex (p=0.177). Regarding the subjects’ age distribution, 251 were under 40 years of age, 236 were between 40 years and 64 years of age, and 98 were over 65 years of age. The highest incidence of PCC was reported in the 40–64 age group (n=73, 30.9%), followed by those under 40 years old (n=62, 24.7%), and those 65 years old or above (n=24, 24.5%). There was also no significant association with PCC according to age group (p=0.245). Forty-six (31.5%) of 146 patients with underlying diseases and 113 (25.7%) of 439 patients without underlying diseases were found to have PCC. Hypertension showed a significant association with PCC (p=0.022), but no association was observed between the presence of at least 1 underlying disease and PCC (p=0.175). Furthermore, 374 of the 585 subjects had initial symptoms at diagnosis. Among the 409 patients who did not have a fever as an initial symptom, 94 (23.0%) were diagnosed with PCC. Conversely, of the 176 patients who had a fever, 65 (36.9%) had PCC (p=0.001). Among the 421 patients without cough and sputum, 98 (23.3%) had PCC, while of the 164 patients with cough and sputum, 61 (37.2%) had PCC (p=0.001). Of the 570 patients who did not experience loss of smell, 150 (26.3%) had PCC, while 9 (60.0%) of the 15 patients who did experience loss of smell had PCC (p=0.004). Among the 489 patients without a sore throat, 123 (25.2%) had PCC, while 36 (37.5%) of the 96 patients with a sore throat had PCC (p=0.013). Of the 500 patients without muscle pain, 125 (25.0%) were diagnosed with PCC, while 34 (40.0%) of the 85 patients with muscle pain had PCC (p=0.004). No significant association was found between PCC and other initial symptoms. The hospitalization period and African ethnicity also showed statistically significant associations with PCC (Table 1).
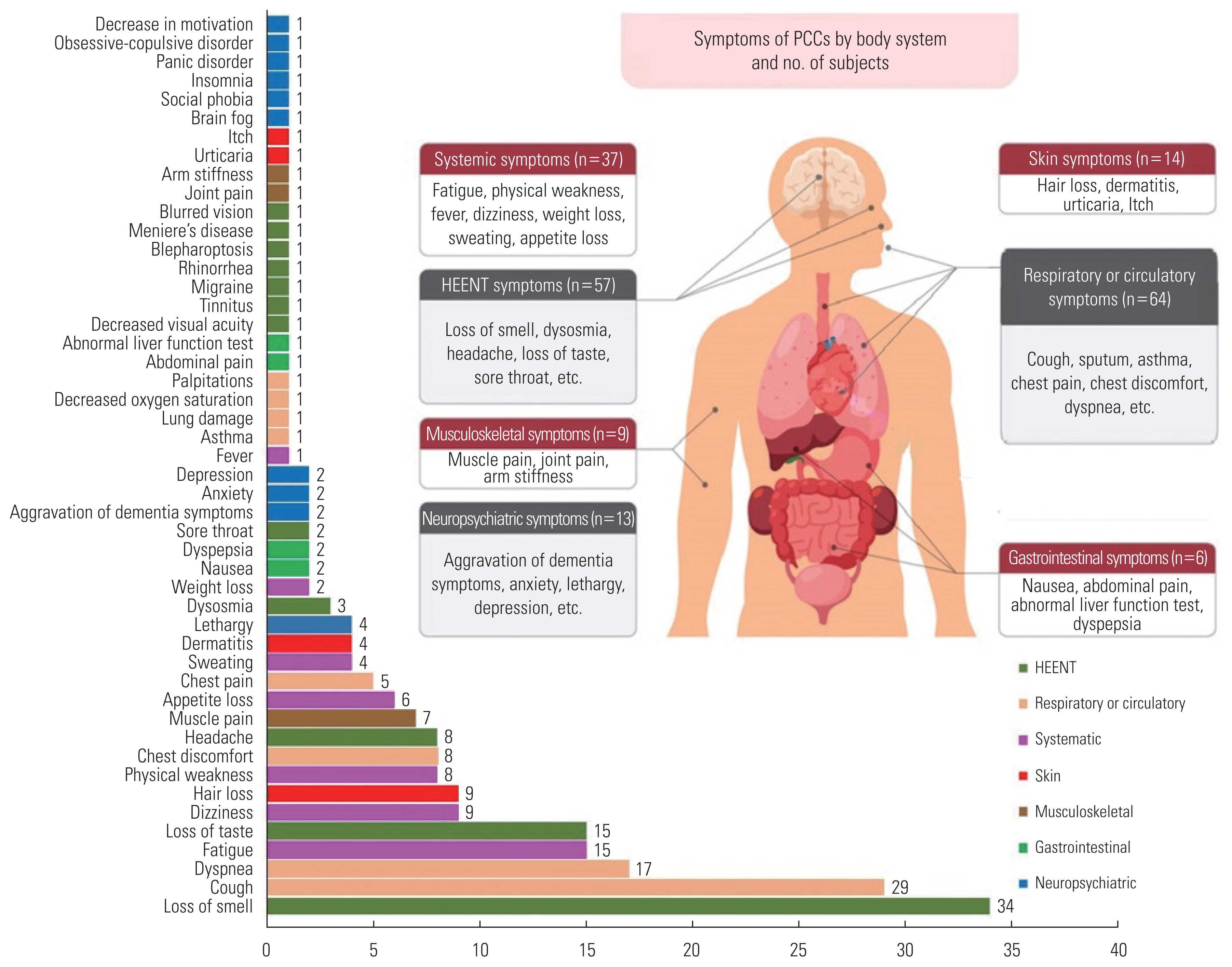
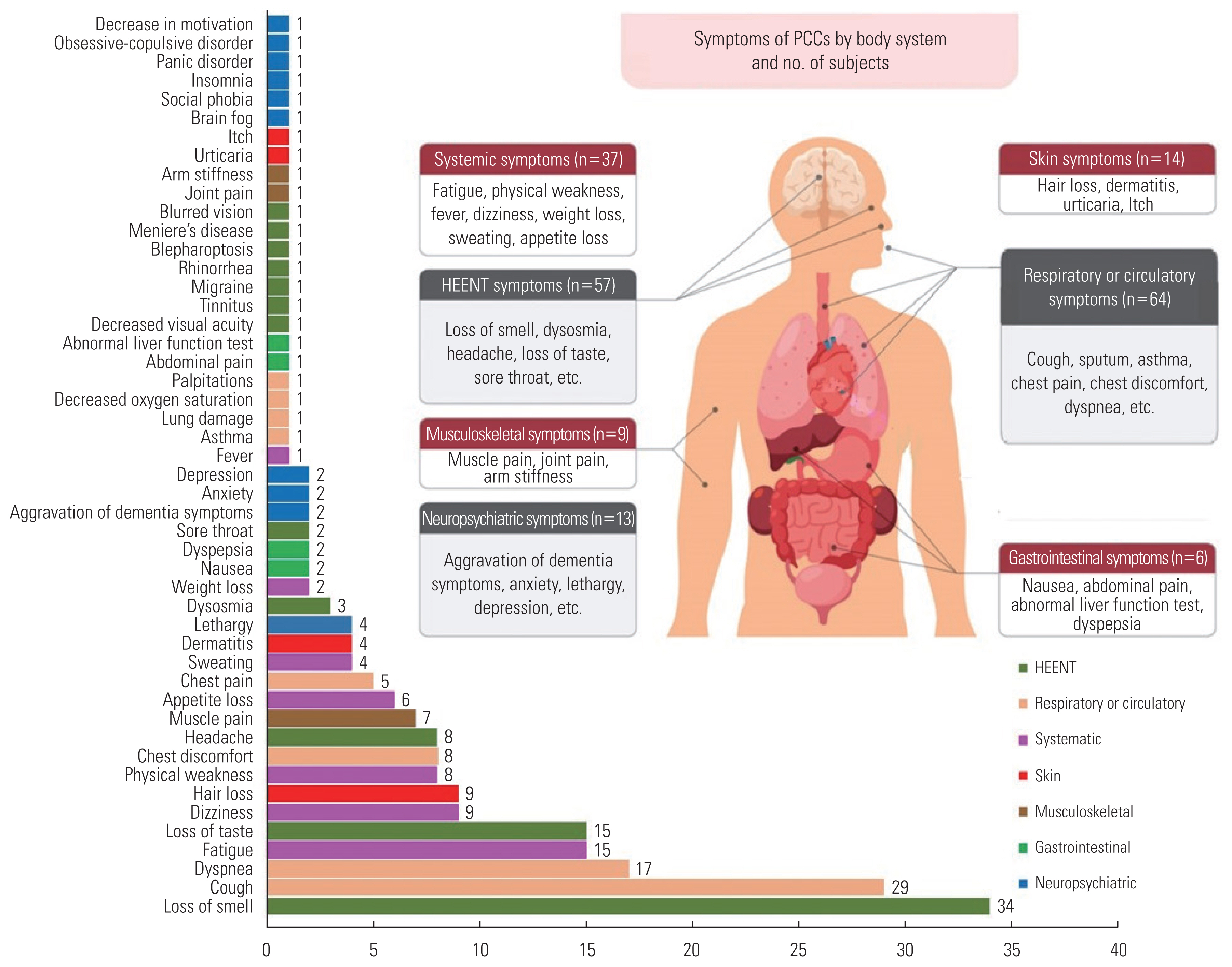
- In total, 49 types of PCC were investigated (Figure 1 and Table 2). Among the 585 subjects, 159 (27.2%) had PCC and 426 (72.8%) did not. PCC was most frequently reported with regard to respiratory or circulatory symptoms (n=64, 40.3%), followed by head/neck/ear/nose and throat symptoms (n=57, 35.8%), systemic symptoms (n=37, 23.3%), skin symptoms (n=14, 8.8%), neuropsychiatric symptoms (n=13, 8.2%), musculoskeletal symptoms (n=9, 5.7%), and gastrointestinal symptoms (n=6, 3.8%). Systemic symptoms included fatigue (n=15, 9.4%), dizziness (n=9, 5.7%), physical weakness (n=8, 5.0%), and appetite loss (n=6, 3.8%). Respiratory or circulatory symptoms included cough (n=29, 18.2%), sputum (n=14, 8.8%), dyspnea (n=17, 10.7%), and chest discomfort (n=8, 5.0%). For the head/neck/ear/nose and throat symptoms, 34 subjects (21.4%) were found to have loss of smell, while 15 patients (9.4%) had loss of taste. In addition, musculoskeletal symptoms such as muscle pain and joint pain, skin symptoms such as hair loss and dermatitis, and neuropsychiatric symptoms such as lethargy, anxiety, and depression, were identified as symptoms of PCC.
- Out of the 211 patients who initially presented no symptoms, 27 (12.8%) eventually developed PCC, with cough and fatigue being the most frequently observed. Among the 374 patients who did exhibit initial symptoms, 132 (35.3%) were diagnosed with PCC. The most common symptoms in this group were loss of smell, cough, dyspnea, loss of taste, fatigue, and sputum production (Supplemental Material 1).
- Upon analyzing the relationship between the length of hospitalization and PCC, we found no statistically significant association with systemic symptoms, or symptoms related to the head, neck, ear, nose, and throat, as well as musculoskeletal symptoms (Table 3). However, within the categories of respiratory and circulatory symptoms, statistically significant associations were observed with sputum and dyspnea. Similarly, among gastrointestinal symptoms, vomiting and nausea were significantly associated with the length of hospitalization (p=0.045, 0.001, and <0.001, respectively).
- The multiple logistic regression performed to examine the relationships between various risk factors and PCC showed no statistically significant findings for sex, age, underlying disease, and ethnicity (Table 4). However, initial symptoms and length of hospitalization showed statistically significant relationships with PCC. Compared to subjects with no initial symptoms, those with initial symptoms of fever or chills, cough or sputum, loss of smell, and sore throat had a higher risk of developing PCC (odds ratio [OR] and 95% confidence interval [CI], 1.79 [1.17 to 2.74]; 1.67 [95% CI, 1.10 to 2.53]; 5.39 [95% CI, 1.80 to 16.09], and 1.69 [95% CI, 1.03 to 2.77], respectively). Compared to subjects with a length of hospitalization of 10 days or less, those with a length of hospitalization of 21 days to 30 days and those with a length of hospitalization of 31 days or more had a statistically significant higher risk of developing PCC (OR [95% CI], 2.32 [95% CI, 1.04 to 5.15] and 5.80 [95% CI, 1.85 to 18.15]).
RESULTS
- This study found that characteristics such as sex, age, underlying disease, and ethnicity did not significantly affect the occurrence of PCC. Patients with initial symptoms of fever or chills, cough or sputum, loss of smell, and sore throat were more likely to develop PCC than patients without initial symptoms. Throughout the study period, all COVID-19 patients were admitted to either residential treatment centers or medical facilities; the longer the hospitalization (21 days or more), the greater the likelihood of experiencing PCC. Furthermore, the study found that various forms of PCC could occur in individuals without initial symptoms (Supplemental Material 1). The study also revealed that initial symptoms of COVID-19, such as cough, loss of smell, headache, and sputum production, could persist until the period identified as PCC.
- A national survey conducted in Malta found that PCC was associated with female sex, hospitalization, and initial symptoms [14]. In this study, it was found that there was no statistically significant association between PCC and sex, which was inconsistent with the Malta study [14]. This result, however, is consistent with a study of the US CDC Morbidity and Mortality Weekly Reports based on electronic medical records, which found no significant association with sex [15]. A study on the non-hospitalized population reported that the number of initial symptoms was related to PCC [16]. This study showed a strong relationship between initial symptoms and PCC, which is consistent with the Malta study but inconsistent with the WHO announcement that initial symptoms are not related to PCC. According to a study by the US CDC COVID-19 Emergency Response Team, PCC was experienced by 20.8% of those aged 18 to 64 and 26.9% of those aged 65 or older with respiratory symptoms and muscle pain [15]. In addition, a study conducted in Korea reported that advanced age was a risk factor for PCC [10], but this is not consistent with our study.
- In this study, 49 PCC symptoms, including fatigue, cough, and shortness of breath, were investigated. These symptoms were identified through an open-ended question and were found in similar proportions and types as in other studies [17–20]. The results are similar to those of previous research, including a systematic review and meta-analysis [1,2,19,21]. One of the outcomes of that study was that a longer hospitalization period was associated with PCC, a finding that is in agreement with the results of this study [16].
- In another study focusing on outpatients and inpatients, it was found that the majority of patients sought hospital care following the onset of initial symptoms. Consequently, the percentage of subjects initially classified as “asymptomatic” among those with PCC was as low as 1.2% [10, 21]. Therefore, in other studies, it has been challenging to examine meaningful data about individuals who did not exhibit initial symptoms, as the study participants were individuals diagnosed with the disease in a hospital setting. However, in Korea, an epidemiological investigation was carried out through an integrated health and disease management system for COVID-19 patients at a local public health center. This approach made it possible to confirm the presence and type of initial symptoms, revealing that 12.8% of those without initial symptoms eventually developed PCC.
- Several potential biological mechanisms for PCC have been identified. These include damage to the autonomic nervous system, inflammation, autoimmunity, endothelial dysfunction, viral persistence, and coagulation activation [22]. However, the precise mechanisms responsible for PCC are not yet fully understood. Further research is required to gain a more comprehensive understanding of these conditions.
- This study has some limitations. First, the survey was only conducted once due to the unplanned nature of the study, which made it impossible to identify any symptoms that may have initially presented, disappeared, and then recurred. A more extensive investigation over a longer period could have allowed for a more detailed analysis of the duration of the sequelae. Second, the survey was not integrated with clinical practice, which may have led to the inclusion of subjective opinions in the classification of initial symptoms and PCC. Symptoms might have been ambiguously categorized by body systems, such as respiratory or circulatory, due to the absence of a clear clinical evaluation. Finally, symptoms that manifest after a certain period may be indicative of other diseases, like influenza, rather than being post-COVID-19 symptoms. This implies the necessity of a differential diagnosis.
- Nevertheless, this study has several implications. It involved subjects whose age distribution mirrored that of the national confirmed cases at the time of the survey (Supplemental Material 2), and participants were surveyed about various initial symptoms and PCC through open-ended questions. In addition, the survey was conducted by telephone on a 1:1 basis for all age groups, unlike a previous study that focused on subjects who were between 17 years to 29 years old [10].
- In conclusion, this study identified specific factors linked to the incidence of PCC. These factors include initial symptoms such as fever, chills, cough, sputum production, loss of smell, and sore throat, as well as extended hospital stays. Moreover, the emergence of PCC in initially asymptomatic patients underscores the necessity for robust guidance and monitoring systems. Given the ongoing COVID-19 epidemic in Korea, there is a notable dearth of research on PCC and its associated risk factors. As such, there is a pressing need for further research in various areas, including the PCC investigation currently being spearheaded by the KDCA.
DISCUSSION
SUPPLEMENTAL MATERIALS
-
CONFLICT OF INTEREST
The authors have no conflicts of interest associated with the material presented in this paper.
-
FUNDING
None.
-
AUTHOR CONTRIBUTIONS
Conceptualization: Jang M, Kwon HJ. Data curation: Jang M, Choi D. Formal analysis: Choi D, Choi J. Funding acquisition: None. Methodology: Jang M, Choi D, Kwon HJ. Project administration: Kwon HJ. Visualization: Jang M. Writing – original draft: Jang M, Choi D. Writing – review & editing: Jang M, Choi D, Choi J, Kwon HJ.
Notes
ACKNOWLEDGEMENTS
| Characteristics | Subjects (n) | PCC | p-value1 | |
|---|---|---|---|---|
|
|
||||
| Yes | No | |||
| Total | 585 | 159 (27.2) | 426 (72.8) | |
|
|
||||
| Sex | 0.177 | |||
| Male | 310 | 77 (24.8) | 233 (75.2) | |
| Female | 275 | 82 (29.8) | 193 (70.2) | |
|
|
||||
| Age (y) | 0.245 | |||
| <40 | 251 | 62 (24.7) | 189 (75.3) | |
| 40–64 | 236 | 73 (30.9) | 163 (69.1) | |
| ≥65 | 98 | 24 (24.5) | 74 (75.5) | |
|
|
||||
| Underlying disease | 0.175 | |||
| No | 439 | 113 (25.7) | 326 (74.3) | |
| Yes | 146 | 46 (31.5) | 100 (68.5) | |
| Hypertension | 0.022 | |||
| No | 493 | 125 (25.4) | 368 (74.6) | |
| Yes | 92 | 34 (37.0) | 58 (63.0) | |
| Hyperlipidemia | 0.116 | |||
| No | 536 | 141 (26.3) | 395 (73.7) | |
| Yes | 49 | 18 (36.7) | 31 (63.3) | |
| Diabetes mellitus | 0.092 | |||
| No | 551 | 154 (27.9) | 397 (72.1) | |
| Yes | 34 | 5 (14.7) | 29 (85.3) | |
| Rhinitis | 0.717 | |||
| No | 580 | 158 (27.2) | 422 (72.8) | |
| Yes | 5 | 1 (20.0) | 4 (80.0) | |
| Athsma | 0.218 | |||
| No | 575 | 158 (27.5) | 417 (72.5) | |
| Yes | 10 | 1 (10.0) | 9 (90.0) | |
| Others | 0.112 | |||
| No | 533 | 140 (26.3) | 393 (73.7) | |
| Yes | 52 | 19 (36.5) | 33 (63.5) | |
|
|
||||
| Initial symptoms at diagnosis | <0.001 | |||
| No | 211 | 27 (12.8) | 184 (87.2) | |
| Yes | 374 | 132 (35.3) | 242 (64.7) | |
| Systemic symptoms | ||||
| Fever/chills | 0.001 | |||
| No | 409 | 94 (23.0) | 315 (77.0) | |
| Yes | 176 | 65 (36.9) | 111 (63.1) | |
| Respiratory symptoms | ||||
| Cough/sputum | 0.001 | |||
| No | 421 | 98 (23.3) | 323 (76.7) | |
| Yes | 164 | 61 (37.2) | 103 (62.8) | |
| Dyspnea | 0.387 | |||
| No | 583 | 159 (27.3) | 424 (72.7) | |
| Yes | 2 | 0 (0.0) | 2 (100) | |
| Head-neck-ear-nose and throat | ||||
| Loss of taste | 0.182 | |||
| No | 571 | 153 (26.8) | 418 (73.2) | |
| Yes | 14 | 6 (42.9) | 8 (57.1) | |
| Loss of smell | 0.004 | |||
| No | 570 | 150 (26.3) | 420 (73.7) | |
| Yes | 15 | 9 (60.0) | 6 (40.0) | |
| Headache | 0.678 | |||
| No | 506 | 136 (26.9) | 370 (73.1) | |
| Yes | 79 | 23 (29.1) | 56 (70.9) | |
| Sore throat | 0.013 | |||
| No | 489 | 123 (25.2) | 366 (74.8) | |
| Yes | 96 | 36 (37.5) | 60 (62.5) | |
| Gastrointestinal symptoms | ||||
| Vomiting/nausea | 0.922 | |||
| No | 581 | 158 (27.2) | 423 (72.8) | |
| Yes | 4 | 1 (25.0) | 3 (75.0) | |
| Diarrhea | 0.358 | |||
| No | 575 | 155 (27.0) | 420 (73.0) | |
| Yes | 10 | 4 (40.0) | 6 (60.0) | |
| Musculoskeletal symptoms | ||||
| Muscle pain | 0.004 | |||
| No | 500 | 125 (25.0) | 375 (75.0) | |
| Yes | 85 | 34 (40.0) | 51 (60.0) | |
|
|
||||
| Hospitalization period (day) | <0.001 | |||
| ≤10 | 199 | 50 (25.1) | 149 (74.9) | |
| 11–20 | 337 | 83 (24.6) | 254 (75.4) | |
| 21–30 | 33 | 16 (48.5) | 17 (51.5) | |
| ≥31 | 16 | 10 (62.5) | 6 (37.5) | |
|
|
||||
| Ethnicity | ||||
| Korean | 470 | 138 (29.4) | 332 (70.6) | - |
| Difference in PCC proportion from the reference | Reference | |||
| Asian | 88 | 18 (20.5) | 70 (79.5) | 0.114 |
| Difference in PCC proportion from the reference | −8.9%p | |||
| European | 7 | 2 (28.6) | 5 (71.4) | 1.000 |
| Difference in PCC proportion from the reference | −0.8%p | |||
| African | 20 | 1 (5.0) | 19 (95.0) | 0.035 |
| Difference in PCC proportion from the reference | −24.4%p | |||
| Types | Classification | Subjects (n=585) |
|---|---|---|
| PCC | No | 426 (72.8) |
| Yes | 159 (27.2) | |
|
|
||
| Systemic symptoms | 37 (23.3) | |
| Fatigue | 15 (9.4) | |
| Appetite loss | 6 (3.8) | |
| Physical weakness | 8 (5.0) | |
| Fever | 1 (0.6) | |
| Dizziness | 9 (5.7) | |
| Weight loss | 2 (1.3) | |
| Sweating | 4 (2.5) | |
|
|
||
| Respiratory or circulatory symptoms | 64 (40.3) | |
| Cough | 29 (18.2) | |
| Sputum | 14 (8.8) | |
| Chest pain | 5 (3.1) | |
| Chest discomfort | 8 (5.0) | |
| Dyspnea | 17 (10.7) | |
| Others1 | 5 (3.1) | |
|
|
||
| Gastrointestinal symptoms | 6 (3.8) | |
| Nausea | 2 (1.3) | |
| Abdominal pain | 1 (0.6) | |
| Abnormal liver function test | 1 (0.6) | |
| Dyspepsia | 2 (1.3) | |
|
|
||
| Head-neck-ear-nose and throat symptoms | 57 (35.8) | |
| Loss of smell | 34 (21.4) | |
| Loss of taste | 15 (9.4) | |
| Dysosmia | 3 (1.9) | |
| Headache | 8 (5.0) | |
| Sore throat | 2 (1.3) | |
| Others2 | 7 (4.4) | |
|
|
||
| Musculoskeletal symptoms | 9 (5.7) | |
| Muscle pain | 7 (4.4) | |
| Joint pain | 1 (0.6) | |
| Arm stiffness | 1 (0.6) | |
|
|
||
| Skin symptoms | 14 (8.8) | |
| Hair loss | 9 (5.7) | |
| Dermatitis | 4 (2.5) | |
| Urticaria | 1 (0.6) | |
| Itch | 1 (0.6) | |
|
|
||
| Neuropsychiatric symptoms | 13 (8.2) | |
| Aggravation of dementia symptoms | 2 (1.3) | |
| Anxiety | 2 (1.3) | |
| Lethargy | 4 (2.5) | |
| Depression | 2 (1.3) | |
| Others3 | 5 (3.1) | |
Values are presented as number (%); The percentage for each symptom was expressed as a proportion among subjects with PCC.
1 Asthma, lung damage, decreased oxygen saturation, and palpitations.
2 Migraine, blepharoptosis, Meniere’s disease, blurred vision, decreased visual acuity, tinnitus, and rhinorrhea.
3 Brain fog, social phobia, insomnia, panic disorder, obsessive-compulsive symptoms, and low motivation.
| PCC | Length of hospitalization (day) | p-value1 | |||
|---|---|---|---|---|---|
|
|
|||||
| ≤10 | 11–20 | 21–30 | ≥31 | ||
| Total | 199 (34.0) | 337 (57.6) | 33 (5.6) | 16 (2.7) | |
|
|
|||||
| Systemic symptoms | 0.890 | ||||
| Yes | 56 (31.8) | 104 (59.1) | 11 (6.3) | 5 (2.8) | |
| No | 143 (35.0) | 233 (57.0) | 22 (5.4) | 11 (2.7) | |
| Fever | 0.980 | ||||
| Yes | 47 (33.6) | 82 (58.6) | 7 (5.0) | 4 (2.9) | |
| No | 152 (34.2) | 255 (57.3) | 26 (5.8) | 12 (2.7) | |
| Chills | 0.374 | ||||
| Yes | 15 (24.6) | 39 (63.9) | 5 (8.2) | 2 (3.3) | |
| No | 184 (35.1) | 298 (56.9) | 28 (5.3) | 14 (2.7) | |
|
|
|||||
| Respiratory or circulatory symptoms | 0.601 | ||||
| Yes | 51 (30.9) | 97 (58.8) | 12 (7.3) | 5 (3.0) | |
| No | 148 (35.2) | 240 (57.1) | 21 (5.0) | 11 (2.6) | |
| Cough | 0.766 | ||||
| Yes | 46 (30.9) | 89 (59.7) | 10 (6.7) | 4 (2.7) | |
| No | 153 (35.1) | 248 (56.9) | 23 (5.3) | 12 (2.8) | |
| Sputum | 0.045 | ||||
| Yes | 13 (32.5) | 21 (52.5) | 6 (15.0) | 0 (0.0) | |
| No | 186 (34.1) | 316 (58.0) | 27 (5.0) | 16 (2.9) | |
| Dyspnea | 0.001 | ||||
| Yes | 0 (0.0) | 1 (50.0) | 0 (0.0) | 1 (50.0) | |
| No | 199 (34.1) | 336 (57.6) | 33 (5.7) | 15 (2.6) | |
|
|
|||||
| Head-neck-ear-nose and throat symptoms | 0.513 | ||||
| Yes | 59 (36.2) | 87 (53.4) | 12 (7.4) | 5 (3.1) | |
| No | 140 (33.2) | 250 (59.2) | 21 (5.0) | 11 (2.6) | |
| Loss of taste | 0.397 | ||||
| Yes | 3 (21.4) | 11 (78.6) | 0 (0.0) | 0 (0.0) | |
| No | 196 (34.3) | 326 (57.1) | 33 (5.8) | 16 (2.8) | |
| Headache | 0.335 | ||||
| Yes | 29 (36.7) | 40 (50.6) | 6 (7.6) | 4 (5.1) | |
| No | 170 (33.6) | 297 (58.7) | 27 (5.3) | 12 (2.4) | |
| Sore throat | 0.642 | ||||
| Yes | 31 (32.3) | 54 (56.3) | 8 (8.3) | 3 (3.1) | |
| No | 168 (34.4) | 283 (57.9) | 25 (5.1) | 13 (2.7) | |
|
|
|||||
| Gastrointestinal symptoms | 0.026 | ||||
| Yes | 3 (25.0) | 6 (50.0) | 1 (8.3) | 2 (16.7) | |
| No | 196 (34.2) | 331 (57.8) | 32 (5.6) | 14 (2.4) | |
| Vomiting and nausea | <0.001 | ||||
| Yes | 0 (0.0) | 2 (50.0) | 0 (0.0) | 2 (50.0) | |
| No | 199 (34.3) | 335 (57.7) | 33 (5.7) | 14 (2.4) | |
| Diarrhea | 0.878 | ||||
| Yes | 3 (30.0) | 6 (60.0) | 1 (10.0) | 0 (0.0) | |
| No | 196 (34.1) | 331 (57.6) | 32 (5.6) | 16 (2.8) | |
|
|
|||||
| Musculoskeletal symptoms | 0.382 | ||||
| Yes | 35 (41.2) | 42 (49.4) | 6 (7.1) | 2 (2.4) | |
| No | 164 (32.8) | 295 (59.0) | 27 (5.4) | 14 (2.8) | |
| Variables | n | PCC, n (%) | aOR (95% CI)1 | p-value |
|---|---|---|---|---|
| Sex | ||||
| Male | 310 | 77 (24.8) | 1.00 (reference) | |
| Female | 275 | 82 (29.8) | 1.28 (0.86, 1.90) | 0.229 |
|
|
||||
| Age (y) | ||||
| <40 | 251 | 62 (24.7) | 1.00 (reference) | |
| 40–64 | 236 | 73 (30.9) | 1.21 (0.78, 1.87) | 0.404 |
| ≥65 | 98 | 24 (24.5) | 0.83 (0.45, 1.53) | 0.553 |
|
|
||||
| Underlying disease | ||||
| None | 439 | 113 (25.7) | 1.00 (reference) | |
| Yes | 146 | 46 (31.5) | 1.22 (0.77, 1.94) | 0.391 |
|
|
||||
| Initial symptoms | ||||
| Fever/chills | ||||
| No | 409 | 94 (23.0) | 1.00 (reference) | |
| Yes | 176 | 65 (36.9) | 1.79 (1.17, 2.74) | 0.007 |
| Cough/sputum | ||||
| No | 421 | 98 (23.3) | 1.00 (reference) | |
| Yes | 164 | 61 (37.2) | 1.67 (1.10, 2.53) | 0.016 |
| Loss of smell | ||||
| No | 570 | 150 (26.3) | 1.00 (reference) | |
| Yes | 15 | 9 (60.0) | 5.39 (1.80, 16.09) | 0.003 |
| Sore throat | ||||
| No | 489 | 123 (25.2) | 1.00 (reference) | |
| Yes | 96 | 36 (37.5) | 1.69 (1.03, 2.77) | 0.036 |
| Muscle pain | ||||
| No | 500 | 125 (25.0) | 1.00 (reference) | |
| Yes | 85 | 34 (40.0) | 1.67 (1.00, 2.80) | 0.051 |
|
|
||||
| Length of hospitalization (day) | ||||
| ≤10 | 199 | 50 (25.1) | 1.00 (reference) | |
| 11–20 | 337 | 83 (24.6) | 0.87 (0.56, 1.35) | 0.529 |
| 21–30 | 33 | 16 (48.5) | 2.32 (1.04, 5.15) | 0.039 |
| ≥31 | 16 | 10 (62.5) | 5.80 (1.85, 18.15) | 0.003 |
|
|
||||
| Ethnicity | ||||
| Korean | 470 | 138 (29.4) | 1.00 (reference) | |
| Asia | 88 | 18 (20.5) | 0.67 (0.37, 1.23) | 0.199 |
| European | 7 | 2 (28.6) | 1.62 (0.29, 9.07) | 0.585 |
| African | 20 | 1 (5.0) | 0.21 (0.03, 1.76) | 0.151 |
- 1. Korea Disease Control and Prevention Agency. Coronavirus infectious diseas-19 response guideline for local governments: 13-1; 2022 [cited 2022 Aug 22] Available from: https://ncov.kdca.go.kr/duBoardList.do?brdId=2&brdGubun=28 (Korean)
- 2. Kim EY, Park SY, Lee SY, Yu JH, Lee H, Ryu B, et al. Cases of outbreaks caused by COVID-19 variant in the Republic of Korea. Public Health Wkly Rep 2021;14(13):742-748. (Korean)
- 3. Park SE. Epidemiology, virology, and clinical features of severe acute respiratory syndrome-coronavirus-2 (SARS-CoV-2; coronavirus disease-19). Clin Exp Pediatr 2020;63(4):119-124ArticlePubMedPMCPDF
- 4. Chang MC, Park YK, Kim BO, Park D. Risk factors for disease progression in COVID-19 patients. BMC Infect Dis 2020;20(1):445ArticlePubMedPMCPDF
- 5. World Health Organization. A clinical case definition of post COVID-19 condition by a Delphi consensus; 6. October. 2021 [cited 2022 Feb 23]. Available from: https://apps.who.int/iris/handle/10665/345824
- 6. Korea Disease Control and Prevention Agency. Notice of post COVID-19 condition; 2022 [cited 2022 May 3] Available from: https://www.kdca.go.kr/gallery.es?mid=a20503020000&bid=0003&act=view&list_no=145681 (Korean)
- 7. Patton LL. Long-COVID and the practice of oral medicine. Oral Surg Oral Med Oral Pathol Oral Radiol 2022;133(2):125-128ArticlePubMedPMC
- 8. National Center for Medical Information and Knowledge. Multi-center observation cohort study on short-and long-term clinical outcomes of COVID19-patients; 2021 [cited 2021 May 13] Available from: https://library.nih.go.kr/ncmiklib/archive/rom/reportView.do?upd_yn=Y&rep_id=RP00012436 (Korean)
- 9. Lee H, Sung HK, Lee D, Choi Y, Lee JY, Lee JY, et al. Comparison of complications after coronavirus disease and seasonal influenza, South Korea. Emerg Infect Dis 2022;28(2):347-353ArticlePubMedPMC
- 10. Kim Y, Bitna-Ha , Kim SW, Chang HH, Kwon KT, Bae S, et al. Post-acute COVID-19 syndrome in patients after 12 months from COVID-19 infection in Korea. BMC Infect Dis 2022;22(1):93ArticlePubMedPMCPDF
- 11. Menges D, Ballouz T, Anagnostopoulos A, Aschmann HE, Domenghino A, Fehr JS, et al. Burden of post-COVID-19 syndrome and implications for healthcare service planning: a population-based cohort study. PLoS One 2021;16(7):e0254523ArticlePubMedPMC
- 12. Zhang X, Wang F, Shen Y, Zhang X, Cen Y, Wang B, et al. Symptoms and health outcomes among survivors of COVID-19 infection 1 year after discharge from hospitals in Wuhan, China. JAMA Netw Open 2021;4(9):e2127403ArticlePubMedPMC
- 13. Augustin M, Schommers P, Stecher M, Dewald F, Gieselmann L, Gruell H, et al. Post-COVID syndrome in non-hospitalised patients with COVID-19: a longitudinal prospective cohort study. Lancet Reg Health Eur 2021;6: 100122ArticlePubMedPMC
- 14. Baruch J, Zahra C, Cardona T, Melillo T. National long COVID impact and risk factors. Public Health 2022;213: 177-180ArticlePubMedPMC
- 15. Bull-Otterson L, Baca S, Saydah S, Boehmer TK, Adjei S, Gray S, et al. Post–COVID conditions among adult COVID-19 survivors aged 18–64 and ≥65 years — United States, March 2020-November 2021. MMWR Morb Mortal Wkly Rep 2022;71(21):713-717ArticlePMC
- 16. Stavem K, Ghanima W, Olsen MK, Gilboe HM, Einvik G. Persistent symptoms 1.5–6 months after COVID-19 in non-hospitalised subjects: a population-based cohort study. Thorax 2021;76(4):405-407ArticlePubMedPMC
- 17. Walitt B, Bartrum E. A clinical primer for the expected and potential post-COVID-19 syndromes. Pain Rep 2021;6(1):e887ArticlePubMedPMC
- 18. Petersen MS, Kristiansen MF, Hanusson KD, Danielsen ME, Á Steig B, Gaini S, et al. Long COVID in the Faroe Islands: a longitudinal study among nonhospitalized patients. Clin Infect Dis 2021;73(11):e4058-e4063ArticlePubMedPMCPDF
- 19. Lopez-Leon S, Wegman-Ostrosky T, Perelman C, Sepulveda R, Rebolledo PA, Cuapio A, et al. More than 50 long-term effects of COVID-19: a systematic review and meta-analysis. Sci Rep 2021;11(1):16144ArticlePubMedPMCPDF
- 20. Yong SJ. Long COVID or post-COVID-19 syndrome: putative pathophysiology, risk factors, and treatments. Infect Dis (Lond) 2021;53(10):737-754ArticlePubMedPMC
- 21. Peghin M, Palese A, Venturini M, De Martino M, Gerussi V, Graziano E, et al. Post-COVID-19 symptoms 6 months after acute infection among hospitalized and non-hospitalized patients. Clin Microbiol Infect 2021;27(10):1507-1513ArticlePubMedPMC
- 22. Castanares-Zapatero D, Chalon P, Kohn L, Dauvrin M, Detollenaere J, Maertens de Noordhout C, et al. Pathophysiology and mechanism of long COVID: a comprehensive review. Ann Med 2022;54(1):1473-1487ArticlePubMedPMC
REFERENCES
Figure & Data
References
Citations

 KSPM
KSPM

 PubReader
PubReader ePub Link
ePub Link Cite
Cite