Articles
- Page Path
- HOME > J Prev Med Public Health > Volume 45(4); 2012 > Article
-
Original Article
Nail DNA and Possible Biomarkers: A Pilot Study - Joshua Park1, Debbie Liang1, Jung Woo Kim2, Yongjun Luo3, Taesheng Huang3, Soo-Young Kim4, Seong-Sil Chang4
-
Journal of Preventive Medicine and Public Health 2012;45(4):235-243.
DOI: https://doi.org/10.3961/jpmph.2012.45.4.235
Published online: July 31, 2012
1Genetic Epidemiology Research Institute, University of California, Irvine, CA, USA.
2Department of Life Science and Technology, Pai Chai University, Daejeon, Korea.
3Division of Human Genetics, Department of Pediatrics, University of California, Irvine, CA, USA.
4Department of Occupational and Environmental Medicine, Eulji University Hospital, Daejeon, Korea.
- Corresponding author: Seong-Sil Chang, MD, PhD. 95 Dunsanseo-ro, Seo-gu, Daejeon 302-799, Korea. Tel: +82-42-611-3000, Fax: +82-42-611-3789, sschang@eulji.ac.kr
- *Park & Liang contributed equally to this work as joint first authors.
• Received: December 25, 2011 • Accepted: February 2, 2012
Copyright © 2012 The Korean Society for Preventive Medicine
This is an Open Access article distributed under the terms of the Creative Commons Attribution Non-Commercial License (http://creativecommons.org/licenses/by-nc/3.0/) which permits unrestricted non-commercial use, distribution, and reproduction in any medium, provided the original work is properly cited.
ABSTRACT
-
Objectives
- Nail has been a substitute DNA source for genotyping. To investigate the integrity and consistency of nail DNA amplification for biomarker study, nail clippings from 12 subjects were collected at monthly intervals. The possibility of longer amplification and existence of GAPDH RNA/protein, were also investigated with three nail samples.
-
Methods
- Three primer sets were designed for quantitative amplification of nuclear and mitochondrial genes and analysis of their consistency. The mean threshold cycles in amplification of the target genes were compared to test the consistency of polymerase chain reaction (PCR) performance among individual factors including age groups, sex, family, the nail source, and by the size of the amplification segments.
-
Results
- The amplification of the target genes from nail DNA showed similar integrity and consistency between the nail sources, and among the serial collections. However, nail DNA from those in their forties showed earlier threshold cycles in amplification than those in their teens or seventies. Mitochondrial DNA (mtDNA) showed better DNA integrity and consistency in amplification of all three targets than did nuclear DNA (nucDNA). Over 9 kb of mtDNA was successfully amplified, and nested quantitative PCR showed reliable copy numbers (%) between the two loci. Reverse transcription PCR for mRNA and immunoblotting for GAPDH protein successfully reflected their corresponding amounts. Regarding the existence of RNA and protein in nails, more effective extraction and detection methods need to be set up to validate the feasibility in biomarker study.
-
Conclusions
- Nail DNA might be a feasible intra-individual monitoring biomarker. Considering integrity and consistency in target amplification, mtDNA would be a better target for biomarker research than nucDNA.
- The identification and monitoring of bio-markers reflect their internal exposures to xenobiotics or effects on target organs, which may be hallmarks of health status and disease progression. Significant amounts of DNA are known to be released into the body fluids from cellular necrosis and apoptosis. Cell free, or circulating DNA in body fluids can provide biologic information and also be used as a marker tracking several human diseases including cancers [1-5]. Appropriate non-invasive access to tissue to monitor biomarkers will maximize efficiency of studies on disease control.
- Nails are modified heavy keratin structures at the ends of the digits, contextures of which sensitively reflect the status of systemic blood supply [6]. Regarding the feasibility of accessing biomarkers from nails, not only protein [7-9] but also DNA [10-14] can be analyzed for molecular epidemiology research, medical diagnostics, and forensic science. It was possible to detect circulatory xenobiotic DNA such as hepatitis B virus in nail clippings among hepatitis B surface antigens positive patients [15]. Therefore, the remarkable advantages of nail specimens might be summarized as follows: 1) Given the abundant blood supply that can reach these exterior tissue structures, nail might contain valuable information such as serum, including the internal body burden of xenobiotic biomolecules and trace elements; 2) no harm and no pain to subjects, as well as high compliance with self-collection would be anticipated for serial monitoring biomarkers; 3) nails are very cost-effective to collect and transport, and can be stored at room temperature; 4) since nails have hard exteriors and layers, these properties serve as barriers and protection against numerous pre-anlaytic factors that may affect the integrity of biomolecules and lead to significant errors in downstream interpretation; 5) regarding the time lag of the growth to the nail edge, a nail edge clippings can still possibly provide recent bio-information. These would be best combined with serum samples, which reflects the present exposure/effect burden of xenobiotics such as metals and trace elements [16].
- There are two DNA sources-nuclear DNA (nucDNA) and mitochondrial DNA (mtDNA)-in the acellular or circulatory compartment of the body. Since mtDNA has a greater number of copies than nucDNA, it is easier to detect and quantify for monitoring genetic biomarkers than nucDNA. However, few studies have investigated the feasibility of these DNA biomarkers for monitoring individual health or disease status. It also remains unexplored whether other biomolecules such as RNA or protein in nails can be detectable or quantified by using conventional methods, reverse transcription polymerase chain reaction (RT-PCR) and immunoblotting.
- Therefore, as a preliminary study exploring the feasibility of nail biomolecules as biomarkers, this study investigated two issues: 1) the feasibility of nail DNA as a genetic biomarker, specifically, whether it provides a consistent reference level among healthy state individuals, and 2) the molecular performance of nail biomolecules, for example, the possible performance of longer segment amplification of DNA and detection of other biomolecules such as RNA and protein.
INTRODUCTION
- Feasibility of Nail DNA
- In the feasibility study of nail DNA for monitoring an individual's health status, the integrity and quantity of DNA were analyzed using nail clippings from 12 volunteer subjects from two related families, under institutional review board study protocol (UCI IRB HS#2008-1697). Three age groups-4 participants each in their teens, forties, and seventies-were recruited as shown Figure 1A. They were related, to minimize genetic and environmental variation and to give more power to detect possible differences in other factors such as age, sex, or among collections. The subjects voluntarily donated their fingernail and toenail clippings three times at one-month interval. The individuals were in homeostatic healthy status for a year before and during the study period. The collected samples were stored at room temperature until the extraction of biomolecules.
- For the DNA integrity study, 72 nail DNA were extracted using a QIAmp DNA microkit (Qiagen, Valencia, CA, USA). The nail samples were stored in paper envelopes for at least two months. Then 10 mg of fingernail and toenail were used for extraction of DNA processing within a week. After the final washing according to the mannual, the DNA was eluted with 70 µL of the buffer from the kits. DNA concentrations were measured using either a Nanodrop-1000 spectrophotometer (Nanodrop Products, Wilmington, DE, USA) or Quant-it™ dsDNA HS assay kit (Invitrogen, Carlsbad, CA, USA). Each 1 µL of nail DNA were plated in each well of 384 well-plates and dried DNA for downstream quantitative PCR (qPCR) .
- To monitor the integrity and consistency of DNA, three primer sets for b-actin and mitochondrially encoded cytochrome C oxidase 1 (mt-Co 1) were designed to amplify 100, 200, and 400 bp products, respectively. For amplification of the three different sizes of b-actin, the common forward primer 5'-CCT GGG TGA GTG GAG ACT GT-3', and 5'-ATG CCT GAG AGG GAA ATG AG-3' as the backward primer for 104 bp amplification, 5'-CAC TGT GTT GGC GTA CAG GT-3' for 192 bp amplification, and 5'-GGA GGA GCA ATG ATC TGA GG-3' for 408 bp amplification were used. For amplification of three different sizes of mt-Co 1 DNA, common forward primer 5'-TTC GCC GAC CGT TGA CTA TTC TCT-3', and the backward primer 5'-TGT GCC TAG GAC TCC AGC TC-3' for 94 bp amplification, the backward primer 5'-TTA CAA ATG CAT GGG CTG TG-3' for 190 bp amplification, and the backward primer 5'-GGT GGG AGT AGT TCC CTG CT-3' for 412 bp amplification were used.
- The qPCR were performed using ABI PRISM 7000 and SYBR Green PCR Master mix (Applied Biosystem, Foster City, CA, USA). The thermocycling condition for mt-Co 1 were 95℃ 10 minutes, 40 cycles of 95℃ 15 seconds, 60℃ 20 seconds, 72℃ 40 seconds, and for dissociation curve, one cycle of 95℃ 15 seconds 60℃ 15 seconds, 95℃ 15 seconds. The thermocycling for b-actin was similar to mt-Co 1 except that the annealing temperature was 55℃.
- Overall amplification of target DNA were successful in 33.8% of nail samples for b-actin and 91.2% for mt-Co 1, when the threshold cycles over 30 were defined as undetermined. Since amplification of b-actin was more than 50% undetermined, b-actin was excluded from further DNA integrity analysis. The mean and standard deviation of threshold cycles between amplifications were compared by product size, age groups, collections, and family groups. The difference between longer segments (400 or 200 bp) and the shortest segments (100 bp) was analyzed for consistency of amplification. A greater difference in threshold cycles or greater undetermined proportion was considered to have less integrity of amplification.
- Molecular Performance of Nail DNA, RNA and Protein
- To assess the longer fragment amplification of mtDNA and the existence of RNA and protein, three patients under the study protocol (UCI IRB HS#2002-2587) donated nail samples. Their health/disease status or other demographic information was unknown. Nail clippings were collected by the subjects and placed in a paper envelope and directly sent to the study investigator. One of the donated blood samples was used as a positive control/reference to compare with those in the nails.
- For longer segment amplification, DNA from 5 mg of three fingernails was extracted using a DNeasy kit (Qiagen). Nail RNA was extracted from 5 mg of three fingernails using an RNeasy Plus kit (Qiagen), according to the manufacturer's manuals.
- The primer sets for the longer template, 9251 bps of mtDNA (569 to 9819), were forward primer 5'-AAC CAA ACC CCA AAG ACA CC-3', and backward primer 5'-GCC AAT AAT GAC GTG GAA GTC C-3' as published previously [17]. TakaRa LA Taq (BR002 AM, Madison, WI, USA) was used for amplification of over 9 kb of mtDNA. The thermocycling condition were 94℃ 1 minute, 30 cycles of 98℃ 10 seconds 68℃ 10 minutes and final extension 72℃ 10 minutes. As for the downstream quantitative PCR, two primer sets for nested quantitative PCR were performed; for mtDNA (3121 to 3319), forward 5'-CAC CCA AGA ACA GGG TTT GT-3', backward 5'-TGG CCA TGG GTA TGT TGT TA-3', and for mtDNA (8825 to 9090) forward 5'-TAA ACC TAG CCA TGG CCA TC-3', backward 5'-AGA GGG AAG GTT AAT GGT TG-3' were used with SYBR Green PCR Master mix (Applied Biosystems). Using the difference in the threshold cycles in nested target amplification between the blood control and each of three individual nails, the relative copy number % of mtDNA in each nails was calculated according to the following formula:
- The primer set for amplifying GAPDH mRNA was forward primer (exon 8) 5'-CAT GAG AAG TAT GAC AAC AGC CT-3', and backward primer (exon 9) 5'-AGT CCT TCC ACG ATA CCA AAG T-3'. The reverse transcriptase PCR was performed with 2.75 µL of total RNA (unknown concentration), 1 µL of each primer (10 pM), 0.2 µL of RNase inhibitor, and 0.05 µL of MultiScribe Reverse Transcriptase (Applied Biosystem), and 5 µL of master mix using the ABI PRISM 7000. The thermocycling conditions for one step RT-PCR were 48℃ 30 minutes, 95℃ 10 minutes, 40 cycles of 95℃ 15 seconds, 60℃ 1 minute, and for the dissociation curve, one cycle of 95℃ 15 seconds 60℃ 15 seconds, 95℃ 15 seconds. Every sample was duplicated. Amplification of the target product was confirmed by the specific Tm peak and gel electrophoresis.
- Nail protein were extracted by a conventional protein lysis method as previously described [18]. From 8.6 to 10.7 mg of nail, protein was extracted through 300 µL of protein lysis buffer, containing 20 mM Tris HCl (pH 8,5), 7 M urea, 5% (v/v) 2-mercaptoethanol, and complete protease inhibitor tablet (Roche, San Francisco, CA, USA). Seven micrograms of extracted protein were separated by sodium dodecyl sulfate polyacrylamide gel electrophoresis (SDS-PAGE) using NuPAGE Novex Bis-Tris 4-12% gel (Invitrogen), and stained with coomassie brillant blue. For western blotting, after SDS-PAGE the protein was transferred to polyvinylidene fluoride membrane (Invitrogen) for 1 hour at 100 V. After blocking with 5% skim milk solution for 1 hour, mouse anti-GAPDH Mab (1:2000 dilutions; Ambion, Austin, TX, USA) was added to 5% milk solution and incubated overnight in a cold room. After washing 3 times with phosphate buffered saline (PBS), horseradish peroxidase conjugated goat anti-mouse antibody (1:10 000 dilution; Sigma-Aldrich, St Louis, MO, USA) was added to a 5% milk solution for 1 hour. After washing 3 times with PBS, the GAPDH protein was detected with western blotting detection reagents (GE Healthcare, Piscataway, NJ, USA).
- Using SPSS version 11.0 (SPSS Inc., Chicago, IL, USA), the mean and standard deviation of the amplification threshold cycle was compared by individual factors using t-test or one way ANOVA with post-hoc comparison using Duncan and Scheffe test, and p-values less than 0.05 were considered to be significantly different in statistical comparison.
METHODS
Subjects
DNA and RNA extraction
Quantitative polymerase chain reaction for long segment amplification
Integrity and consistency in target amplification
Subjects
DNA and RNA extraction
Amplification of longer segments of DNA and estimation of relative copy number of nested segments
Detection of GAPDH mRNA: quantitative reverse transcriptase polymerase chain reaction
Immunoblotting for nail GAPDH protein
Statistical analysis
- DNA Yield
- The yield of DNA from approximately 10 mg of finger or toenail was 687.4 ng (9.82 ng/µL×70 µL) according to an optical density (OD) reading from the Nanodrop, or 29.4 ng (0.42 ng/µL×70 µL) according to a Picogreen staining reading. The concentration of DNA read by the OD measurement using the Nanodrop was greater in fingernail, but DNA concentration from the Picogreen reading was greater in toenail (Table 1).
- DNA Integrity of Different Target Amplification Sizes
- Regarding 72 nail DNA samples' PCR performance, 27 (37.5%), 56 (77.8%), and 60 (83.3%) samples were classified as undetermined for the amplification of 100, 200, and 400 bp of b-actin, respectively. In comparison, 6 (8.3%), 5 (6.9%), and 8 (11.1%), respectively, were classified as undetermined for the corresponding size amplification of mt-Co 1. The mean threshold cycles for 100, 200, and 400 bp mt-Co 1 amplification were 19.76±1.53, 20.41±2.01, and 23.93±2.46. The mean threshold cycle for 100 bp b-actin was 27.87±1.03, which delayed 8.1 threshold cycles from mt-Co 1 100 bp amplification, reflecting the fact that 274 times more copies of mtDNA than b-actin existed in nails (Table 2). The difference between 100 and 200 bp amplification was 0.65 cycles, and that between 100 and 400 bp was 3.17 cycles (Table 2). The threshold cycles of DNA amplification between 100 and 200 bp amplification were not different, but the 400 bp amplifications were significantly delayed beyond 100 or 200 bp amplification (p<0.05). Less than 200 bp of amplification showed no decreased integrity in nail DNA PCR performance. Since b-actin showed much less integrity than mt-Co 1, mitochondrial DNA was shown to be more feasible for monitoring genetic biomarker through serial collection than nuclear DNA.
- DNA Integrity According to Individual Factors
- The threshold cycles of mt-Co 1 were not significantly different based on the source location of the fingernail and toenail, between males and females, or among the serial collections. But all three sizes of mt-Co 1 amplification in persons in their forties showed earlier threshold cycles than those from persons in their teens or seventies. In 100 and 200 bp amplification of mt-Co 1, all 12 family members showed similar threshold cycles, with no significant difference in statistics. However, those in the 9 year-old subject and 11 year-old subject and the 400 bp segment in the 69 year-old subject in family 2, showed significantly delayed threshold cycles compared to those of other subjects. There was a significant difference in the threshold cycles between families; family 1 also showed earlier threshold cycles in all three sizes of mt-Co 1 amplification than did family 2 (Figure 1B). The 69 year-old subject in family 2 showed significantly great difference between 200 or 400 and 100 bp amplification, compared to the other 11 subjects. Considering all this, 400 bp amplification showed less integrity and consistency than 100 or 200 bp amplification (Figure 1C).
- 9.2 kb Amplification of Mitochondrial DNA
- From three samples of nail DNA, 9.251 kb (mt569 to mt9819) was successfully amplified (Figure 2). Using 1 µL of product, downstream nest qPCR was performed. The amount of DNA used for qPCR were ranged from 143.6 to 150.0 ng in 1 µL of nail DNA.
- The threshold cycles of blood mtDNA targeted for (3121 to 3319) and (8825 to 9090) were 9.07 and 9.87, while the mean threshold cycles of the three nails targeted for the two segments were 12.6±1.6 and 13.4±1.7, respectively. The difference in the threshold cycles for downstream amplification were 4.71, 4.26, and 1.64 cycles delayed in DNA from nail 1, nail 2, and nail 3, when they were subtracted by the threshold cycles of blood DNA. Considering one delayed cycle to have half of the copy number of the reference blood, estimated relative copy number % of mtDNA mtDNA in nail 1, nail 2, and nail 3 were 3.82%, 5.22%, and 32.19% of the blood reference, respectively. The amount of mtDNA in nail 3 was higher than in nail1 or nail 2. The band intensities on the gel were respectively matched to these estimations of the amount of DNA (Table 3).
- GAPDH RNA and Protein in Nail
- Glyceraldehyde 3 phosphate dehydrogenase mRNA (113 bp of exon8-exon9) was successfully amplified from two samples (Figure 3A), and two samples of GAPDH protein showed positive bands in immunoblotting among three nails (Figure 3B). In nail 1, both GAPDH RNA and protein failed to show the bands on the gel or immunoblotting membrane. The amount of GAPDH RNA and protein in nail 2 showed thicker bands than those of nail 3 (Figure 3). From the same amount of RNA extracts and total protein, the expression profiles were quite different among the three subjects.
RESULTS
- DNA extracted from toenails [13], or fingernails [14,19-21] has been used for genotyping and identification of individuals in the fields of genetic epidemiology and forensic science. Regarding the efficiency of nail DNA extraction, complete digestion of protein is the critical step for obtaining better quality and quantity. In one study, the yield using proteinase K only ranged from 220 to 384 ng from 5 mg of nail clippings, meanwhile using protease from Cucumis melo with EDTA increased the yield up to 1 µg [10]. Other researchers extracted approximately 1 µg of DNA from a 10 mg fingernail by adding 2 mg/L urea to their lysis buffer [14]. In this study, we used a commercial kit without any substitution of enzymes nor additional chemicals. Using fine chopping of the raw material and maximum incubation time for the lysis step as recommended by the manual, the yield of nail DNA in this study was 687.4 ng (473 to 905 ng) from a 10 mg nail sample.
- The nucleic acid-based assays for the evaluation of body fluids provides a very practical method for assessing health status [22], such as the diagnosis of fetal genes in early intrauterine life, the detection and monitoring of tumors, diabetes mellitus, trauma, stroke [23-25], and endometriosis [26], and multimodal therapy effect [27]. Trace elements or heavy metals in nails [28-33] are also analyzed for monitoring the occupational and environmental exposure burden of the human body. Nail might be a promising specimen for assessing numerous biomarkers for monitoring the xenobiotic exposure of and effect burdens on the human body. Usually, the most problematic issue in biomarker monitoring is differentiating the disease specificity from normal background variation [34]. Those biomarkers that can provide consistent baseline levels would be good candidates for monitoring the whole spectrum of individual health/disease. Their performance should be investigated to determine whether the outcomes are comparable among various specimens such serum, other body fluids or nails during the whole spectrum of specific diseases. The limitation of the current study is that it was not possible to comprehensively cover these aspects of the feasibility of biomarker studies.
- As a preliminary biomarker study, we described the change of nucDNA and mtDNA PCR performance during a short-term period of monitoring healthy individuals. According to our results, mtDNA, specifically in nail, showed measurable and consistent amounts of the change of threshold cycles within 2 cycles of difference in 100 or 200 bp amplification. Statistically significant differences in the PCR performance of mtDNA amplification came from age and family factors, and not from the collection methods or sources (finger versus toenail), even though the members of each of the two families were close relatives to each other. This study showed a possibility of setting up individual reference values for the amplification performance of target mtDNA in nails for health status monitoring.
- The amount and genotype of mtDNA are associated with health status and change of disease status. Whether age group based longer interval monitoring or sporadic episodes of individual diseases could change the amount and downstream PCR performance of whole mtDNA or other specific biomolecules in nail also remains for coming studies.
- In our molecular performance study, we successfully amplified over 9 kb segments of mtDNA using nail DNA, since mtDNA has better integrity, and 100 to 1000 times more abundance than nucDNA. We showed there was a detectable amount of nucDNA, mRNA and protein in the nail samples. Regarding the existence of other RNA or protein in nail samples, some studies have shown that xenobiotic RNA such as dermatophytes' mRNA exist in infected nails [35], and small internal proteins also exist in nails [7]. In this study, the amount of GAPDH mRNA and target protein varied widely among the three unrelated subjects. Even though the detection quantity of mRNA and protein of the same subject's nail corresponded with each other on the gel and immunoblotting membrane; nail 2 contained the largest quantity of mRNA and protein among the three nails. In nail 1, both biomolecules were undetectable. Using the conventional extraction method, the integrity of the nucDNA in nail showed limited amplification: The failure rate of amplification longer than 200 bp was over 50%. However, mtDNA in the nails showed much less limitation in amplification up to 9 Kb. Considering that nails are mainly composed of hard keratin, more effective digestion, extraction and measurement methods might need to be developed for the feasible performance of nucDNA, RNA and protein profiles in nail biomarker research.
DISCUSSION
ACKNOWLEDGEMENTS
- 1. Lecomte T, Berger A, Zinzindohoue F, Micard S, Landi B, Blons H, et al. Detection of free-circulating tumor-associated DNA in plasma of colorectal cancer patients and its association with prognosis. Int J Cancer 2002;100(5):542-548. 12124803ArticlePubMed
- 2. Swisher EM, Wollan M, Mahtani SM, Willner JB, Garcia R, Goff BA, et al. Tumor-specific p53 sequences in blood and peritoneal fluid of women with epithelial ovarian cancer. Am J Obstet Gynecol 2005;193(3 Pt 1):662-667. 16150257ArticlePubMed
- 3. Taback B, Hoon DS. Circulating nucleic acids and proteomics of plasma/serum: clinical utility. Ann N Y Acad Sci 2004;1022: 1-8. 15251932ArticlePubMed
- 4. Salani R, Davidson B, Fiegl M, Marth C, Muller-Holzner E, Gastl G, et al. Measurement of cyclin E genomic copy number and strand length in cell-free DNA distinguish malignant versus benign effusions. Clin Cancer Res 2007;13(19):5805-5809. 17908972ArticlePubMed
- 5. Herrera LJ, Raja S, Gooding WE, El-Hefnawy T, Kelly L, Luketich JD, et al. Quantitative analysis of circulating plasma DNA as a tumor marker in thoracic malignancies. Clin Chem 2005;51(1):113-118. 15539466ArticlePubMed
- 6. Trueb RM, Hurlimann AF, Burg G. Periarteritis nodosa cutanea. 20 years follow-up with leg pain, Raynaud syndrome and nail changes. Hautarzt 1995;46(8):568-572. (German). 7558827ArticlePubMed
- 7. Inoue T, Kizawa K, Ito M. Characterization of soluble protein extracts from keratinized tissues: identification of ubiquitin universally distributed in hair, nail, and stratum corneum. Biosci Biotechnol Biochem 2001;65(4):895-900. 11388470ArticlePubMed
- 8. Dekio S, Jidoi J. Comparison of human hair and nail low-sulfur protein compositions on two-dimensional electrophoresis. J Dermatol 1989;16(4):284-288. 2600266ArticlePubMed
- 9. Oimomi M, Hatanaka H, Ishikawa K, Kubota S, Yoshimura Y, Baba S. Increased fructose-lysine of nail protein in diabetic patients. Klin Wochenschr 1984;62(10):477-478. 6431176ArticlePubMed
- 10. Yoshida-Yamamoto S, Nishimura S, Okuno T, Rakuman M, Takii Y. Efficient DNA extraction from nail clippings using the protease solution from Cucumis melo. Mol Biotechnol 2010;46(1):41-48. 20306236ArticlePubMed
- 11. Nie SJ, Yang YM, Tang WR, Xu BY, Jing Q, Xiao CJ. Extraction and analysis of nuclear DNA from free margin of nail material. Yi Chuan 2007;29(11):1373-1377. (Chinese). 17989048ArticlePubMed
- 12. Anderson TD, Ross JP, Roby RK, Lee DA, Holland MM. A validation study for the extraction and analysis of DNA from human nail material and its application to forensic casework. J Forensic Sci 1999;44(5):1053-1056. 10486958ArticlePubMed
- 13. Van Breda SG, Hogervorst JG, Schouten LJ, Knaapen AM, van Delft JH, Goldbohm RA, et al. Toenails: an easily accessible and long-term stable source of DNA for genetic analyses in large-scale epidemiological studies. Clin Chem 2007;53(6):1168-1170. 17517594ArticlePubMed
- 14. Nakashima M, Tsuda M, Kinoshita A, Kishino T, Kondo S, Shimokawa O, et al. Precision of high-throughput single-nucleotide polymorphism genotyping with fingernail DNA: comparison with blood DNA. Clin Chem 2008;54(10):1746-1748. 18824581ArticlePubMed
- 15. Nishiyori A, Fukuda K, Sata M, Tanikawa K. HBV DNA can be detected from nail clippings of HBs Ag positive patients. Kurume Med J 2000;47(1):95-96. 10812896ArticlePubMed
- 16. Rogers MA, Thomas DB, Davis S, Weiss NS, Vaughan TL, Nevissi AE. A case-control study of oral cancer and pre-diagnostic concentrations of selenium and zinc in nail tissue. Int J Cancer 1991;48(2):182-188. 2019465ArticlePubMed
- 17. Tang S, Huang T. Characterization of mitochondrial DNA heteroplasmy using a parallel sequencing system. Biotechniques 2010;48(4):287-296. 20569205ArticlePubMed
- 18. Fujii T, Murai S, Ohkawa K, Hirai T. Effects of human hair and nail proteins and their films on rat mast cells. J Mater Sci Mater Med 2008;19(6):2335-2342. 18157509ArticlePubMed
- 19. Cline RE, Laurent NM, Foran DR. The fingernails of Mary Sullivan: developing reliable methods for selectively isolating endogenous and exogenous DNA from evidence. J Forensic Sci 2003;48(2):328-333. 12664990ArticlePubMed
- 20. Tanigawara Y, Kita T, Hirono M, Sakaeda T, Komada F, Okumura K. Identification of N-acetyltransferase 2 and CYP2C19 genotypes for hair, buccal cell swabs, or fingernails compared with blood. Ther Drug Monit 2001;23(4):341-346. 11477314ArticlePubMed
- 21. Wiegand P, Bajanowski T, Brinkmann B. DNA typing of debris from fingernails. Int J Legal Med 1993;106(2):81-83. 8217869ArticlePubMed
- 22. Taback B, Hoon DS. Circulating nucleic acids in plasma and serum: past, present and future. Curr Opin Mol Ther 2004;6(3):273-278. 15264429PubMed
- 23. Giasuddin AS, Jhuma KA, Haq AM. Applications of free circulating nucleic acids in clinical medicine: recent advances. Bangladesh Med Res Counc Bull 2008;34(1):26-32. 18783074ArticlePubMed
- 24. Tong YK, Lo YM. Diagnostic developments involving cell-free (circulating) nucleic acids. Clin Chim Acta 2006;363(1-2):187-196. 16126188ArticlePubMed
- 25. Pinzani P, Salvianti F, Pazzagli M, Orlando C. Circulating nucleic acids in cancer and pregnancy. Methods 2010;50(4):302-307. 20146940ArticlePubMed
- 26. Zachariah R, Schmid S, Radpour R, Buerki N, Fan AX, Hahn S, et al. Circulating cell-free DNA as a potential biomarker for minimal and mild endometriosis. Reprod Biomed Online 2009;18(3):407-411. 19298741ArticlePubMed
- 27. Zitt M, Muller HM, Rochel M, Schwendinger V, Zitt M, Goebel G, et al. Circulating cell-free DNA in plasma of locally advanced rectal cancer patients undergoing preoperative chemoradiation: a potential diagnostic tool for therapy monitoring. Dis Markers 2008;25(3):159-165. 19096128ArticlePubMedPMCPDF
- 28. Lakshmi Priya MD, Geetha A. Level of trace elements (copper, zinc, magnesium and selenium) and toxic elements (lead and mercury) in the hair and nail of children with autism. Biol Trace Elem Res 2011;142(2):148-158. 20625937ArticlePubMed
- 29. Ilhan A, Ozerol E, Gulec M, Isik B, Ilhan N, Akyol O. The comparison of nail and serum trace elements in patients with epilepsy and healthy subjects. Prog Neuropsychopharmacol Biol Psychiatry 2004;28(1):99-104. 14687863ArticlePubMed
- 30. Hopps HC. The biologic bases for using hair and nail for analyses of trace elements. Sci Total Environ 1977;7(1):71-89. 319530ArticlePubMed
- 31. Hussein Were F, Njue W, Murungi J, Wanjau R. Use of human nails as bio-indicators of heavy metals environmental exposure among school age children in Kenya. Sci Total Environ 2008;393(2-3):376-384. 18243277ArticlePubMed
- 32. Nowak B. Occurrence of heavy metals, sodium, calcium, and potassium in human hair, teeth, and nails. Biol Trace Elem Res 1996;52(1):11-22. 8860662ArticlePubMed
- 33. Mandal BK, Ogra Y, Suzuki KT. Speciation of arsenic in human nail and hair from arsenic-affected area by HPLC-inductively coupled argon plasma mass spectrometry. Toxicol Appl Pharmacol 2003;189(2):73-83. 12781625ArticlePubMed
- 34. Barker PE, Murthy M. Biomarker validation for aging: lessons from mtDNA heteroplasmy analyses in early cancer detection. Biomark Insights 2009;4: 165-179. 20029650ArticlePubMedPMCPDF
- 35. Tsuboi R, Okeke CN, Inoue A, Yamazaki M, Hiruma M, Ogawa H. Identification and viability assessment of dermatophytes infecting nail based on quantitative PCR of dermatophyte actin (ACT) mRNA. Nihon Ishinkin Gakkai Zasshi 2002;43(2):91-93. (Japanese). 12040366ArticlePubMed
REFERENCES
Figure 1Family pedigree and comparison of target amplification among 12 subjects. (A) Each family has three generations composed of the grandparents (69 to 74 years old), parents (43 to 46 years old) and two children (7 to 11 years old). The mothers in the two families were daughters of the same parents. (B) The threshold cycles of three different amplifications among the two children and 400 bp amplification of the grandmother in family 2 showed significantly delayed performance in amplification (ANOVA/Duncan Scheffe among the twelve subjects). Family 2 in total showed significantly delayed threshold cycles in amplification of all three segments compared to those of family 1 in total (t-test between the families). (C) The difference in the threshold cycles between the longer and shorter segments showed a larger standard deviation than the threshold cycles among twelve subjects. Among the 12 subjects, the grandmother of the family 1 showed significantly greater difference in threshold cycles than the other subjects (ANOVA/Duncan Scheffe). Family 2 in total showed a greater difference in both ratios of the two sizes comparison than those of family 1 in total (t-test between the families). mt-Co 1, mitochondrially encoded cytochrome C oxidase 1.
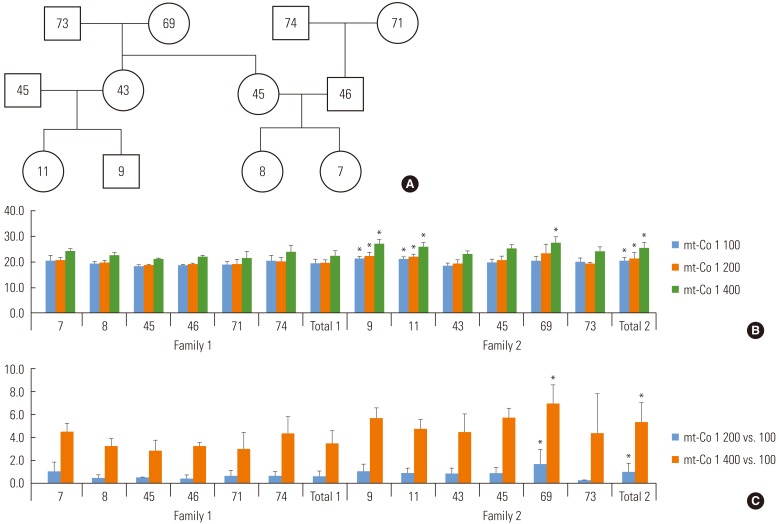

Figure 2Nested target amplification of mitochondrial DNA (mtDNA). (A) Over 9.2 kb was successfully amplified in three nail DNA samples and the blood(+) control. Nested polymerase chain reaction for mtDNA segments for 3121 to 3319 (B,D) and 8825 to 9090 (C,E) were also successfully quantified in three nail DNA samples and the blood sample.
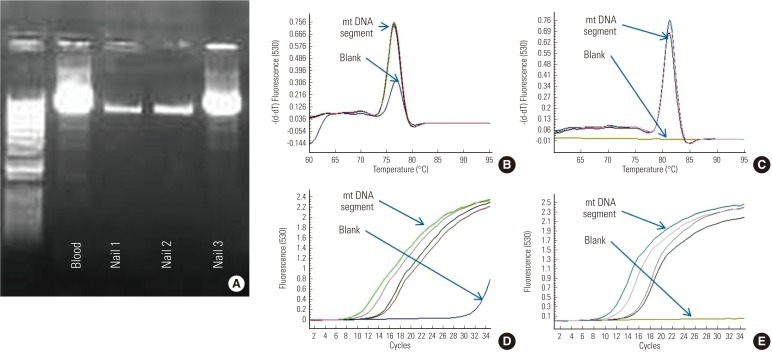

Figure 3GAPDH mRNA and protein. (A) GAPDH mRNA between exon8 and exon9 were successfully amplified in two of the three nail RNA samples. The band of mRNA in nail 2 was brighter than in nail 3, but nail 1 failed to show an mRNA band on the gel. (B) Commessa staining of the nail protein extracts and immunoblotting for GAPDH protein. The amount of GAPDH in nail 2 was more than that of nail 3, and nail 1 failed to show the target protein band on the immunoblotting.

Table 1.Concentration of DNA (ng/μL) in 70 μL of elution buffer
Table 2.Threshold cycles of quantitative polymerase chain reaction
| mt-Co 1 100 | mt-Co 1 200 | mt-Co 1 400 | b-actin 100 | b-actin 200 | b-actin 400 | ||
|---|---|---|---|---|---|---|---|
| Total | n1 | 66 | 67 | 64 | 45 | 16 | 12 |
| Mean | 19.76 | 20.41 | 23.93 | 27.87 | 28.92 | 28.86 | |
| SD | 1.53 | 2.01 | 2.46 | 1.03 | 0.95 | 1.13 | |
| Source2 (p-value) | 0.23 | 0.64 | 0.18 | 0.66 | 0.07 | 0.07 | |
| Fingernail | n | 35 | 32 | 31 | 18 | 6 | 6 |
| Mean | 19.94 | 20.28 | 24.36 | 27.94 | 29.47 | 29.50 | |
| SD | 1.76 | 1.62 | 2.43 | 0.71 | 0.47 | 0.09 | |
| Toenail | n | 31 | 35 | 33 | 27 | 10 | 6 |
| Mean | 19.54 | 20.51 | 23.52 | 27.81 | 28.58 | 28.22 | |
| SD | 1.22 | 2.32 | 2.45 | 1.21 | 1.0 | 1.35 | |
| Collections3 (p-value) | 0.56 | 0.89 | 0.75 | 0.40 | 0.90 | 0.84 | |
| 1st collection | n | 23 | 22 | 22 | 12 | 6 | 4 |
| Mean | 20.03 | 20.48 | 24.21 | 27.62 | 29.05 | 29.09 | |
| SD | 1.59 | 1.67 | 2.66 | 1.05 | 1.09 | 1.24 | |
| 2nd collection | n | 22 | 22 | 21 | 16 | 7 | 5 |
| Mean | 19.67 | 20.50 | 23.63 | 27.78 | 28.79 | 28.88 | |
| SD | 1.70 | 2.40 | 2.30 | 1.00 | 0.63 | 0.93 | |
| 3rd collection | n | 21 | 23 | 21 | 17 | 3 | 3 |
| Mean | 19.54 | 20.24 | 23.94 | 28.13 | 28.97 | 28.53 | |
| SD | 1.30 | 1.97 | 2.50 | 1.06 | 1.56 | 1.64 | |
| Age group3 (p-value) | <0.001 | 0.01 | 0.009 | <0.001 | 0.12 | 0.28 | |
| Teens | n | 22 | 22 | 21 | 12 | 1 | 1 |
| Mean | 20.58 | 21.17 | 24.90 | 28.39 | 29.76 | 29.46 | |
| SD | 1.42 | 1.43 | 2.15 | 0.65 | . | . | |
| Fourties | n | 24 | 23 | 23 | 20 | 7 | 4 |
| Mean | 18.85 | 19.49 | 22.74 | 28.19 | 29.35 | 29.51 | |
| SD | 1.02 | 1.31 | 1.79 | 0.76 | 0.41 | 0.41 | |
| Seventies | n | 20 | 22 | 20 | 13 | 8 | 7 |
| Mean | 19.94 | 20.60 | 24.28 | 26.89 | 28.44 | 28.41 | |
| SD | 1.65 | 2.69 | 2.94 | 1.06 | 1.11 | 1.3 | |
| Family2 (p-value) | 0.03 | <0.001 | <0.001 | 0.20 | 0.79 | 0.38 | |
| Faimily 1 | n | 35 | 34 | 32 | 30 | 12 | 9 |
| Mean | 19.36 | 19.58 | 22.46 | 27.71 | 28.88 | 28.69 | |
| SD | 1.55 | 1.18 | 1.77 | 0.92 | 1.09 | 1.27 | |
| Family 2 | n | 31 | 33 | 32 | 15 | 4 | 3 |
| Mean | 20.20 | 21.26 | 25.41 | 28.18 | 29.04 | 29.39 | |
| SD | 1.42 | 2.32 | 2.18 | 1.20 | 0.33 | 0.17 |
Table 3.Threshold cycles and relative copy number percentage among nested quantitative polymerase chain reaction products of two loci from 9.2 kb amplification product
| Blank control | Blood (+) control | Nail 1 | Nail 2 | Nail 3 | |
|---|---|---|---|---|---|
| Location 1 | >30 | 9.07 | 13.78 | 13.23 | 10.69 |
| Location 2 | Not detected | 9.87 | 14.58 | 14.23 | 11.52 |
| RCN (%)1 of location 1 | 100 | 3.82 | 5.59 | 32.53 | |
| RCN (%) of location 2 | 100 | 3.82 | 4.86 | 31.86 | |
| Mean of RCN (%) of two loci | 100 | 3.82 | 5.22 | 32.19 |
Figure & Data
References
Citations
Citations to this article as recorded by 

- An Investigation for Heavy Metals’ Contamination in Farmers’ Fingernails: Case Study in Libya
Aiman M. Bobaker, Intisar Alakili, Elrashied E. Elkhidir, Sukiman B. Sarmani, Zaher Mundher Yaseen, Mahmood Ahmed
Journal of Chemistry.2022; 2022: 1. CrossRef - Biobanking in Molecular Biomarker Research for the Early Detection of Cancer
Kim Lommen, Selena Odeh, Chiel C. de Theije, Kim M. Smits
Cancers.2020; 12(4): 776. CrossRef - Toenail as Non-invasive Biomarker in Metal Toxicity Measurement of Welding Fumes Exposure - A Review
S F Z Bakri, A Hariri, N F Ma’arop, N S A W Hussin
IOP Conference Series: Materials Science and Engineering.2017; 165(1): 012019. CrossRef - High-Quality DNA from Fingernails for Genetic Analysis
Sandra Preuner, Martin Danzer, Johannes Pröll, Ulrike Pötschger, Anita Lawitschka, Christian Gabriel, Thomas Lion
The Journal of Molecular Diagnostics.2014; 16(4): 459. CrossRef - Detection of short tandem repeat polymorphisms from human nails using direct polymerase chain reaction method
Jian Tie, Seisaku Uchigasaki
ELECTROPHORESIS.2014; 35(21-22): 3188. CrossRef - DNA from Nails for Genetic Analyses in Large-Scale Epidemiologic Studies
Janneke G.F. Hogervorst, Roger W.L. Godschalk, Piet A. van den Brandt, Matty P. Weijenberg, Bas A.J. Verhage, Leonie Jonkers, Joy Goessens, Colinda C.J.M. Simons, Joris R. Vermeesch, Frederik J. van Schooten, Leo J. Schouten
Cancer Epidemiology, Biomarkers & Prevention.2014; 23(12): 2703. CrossRef
 KSPM
KSPM


 PubReader
PubReader ePub Link
ePub Link Cite
Cite




