Articles
- Page Path
- HOME > J Prev Med Public Health > Volume 48(1); 2015 > Article
-
Review
Alcohol as a Risk Factor for Cancer: Existing Evidence in a Global Perspective - Nina Roswall1,2, Elisabete Weiderpass2-5
-
Journal of Preventive Medicine and Public Health 2015;48(1):1-9.
DOI: https://doi.org/10.3961/jpmph.14.052
Published online: January 27, 2015
1Danish Cancer Society Research Center, Copenhagen, Denmark
2Department of Medical Epidemiology and Biostatistics, Karolinska Institutet, Stockholm, Sweden
3Genetic Epidemiology Group, Folkhälsan Research Center, Helsinki, Finland
4The Cancer Registry of Norway, Oslo, Norway
5Department of Community Medicine, UiT-The Arctic University of Norway, Tromsö, Norway
- Corresponding author: Elisabete Weiderpass, MD, PhD PO Box 281, SE-171 77 Stockholm, Sweden Tel: +46-8-524-823-65, Fax: +46-8-31-11-01 E-mail: Elisabete.Weiderpass.Vainio@ki.se
Copyright © 2015 The Korean Society for Preventive Medicine
This is an Open Access article distributed under the terms of the Creative Commons Attribution Non-Commercial License (http://creativecommons.org/licenses/bync/3.0/) which permits unrestricted non-commercial use, distribution, and reproduction in any medium, provided the original work is properly cited.
ABSTRACT
- The purpose of the present review is to give an overview of the association between alcohol intake and the risk of developing cancer. Two large-scale expert reports; the World Cancer Research Fund (WCRF)/American Institute of Cancer Research (AICR) report from 2007, including its continuous update project, and the International Agency for Research of Cancer (IARC) monograph from 2012 have extensively reviewed this association in the last decade. We summarize and compare their findings, as well as relate these to the public health impact, with a particular focus on region-specific drinking patterns and disease tendencies. Our findings show that alcohol intake is strongly linked to the risk of developing cancers of the oral cavity, pharynx, larynx, oesophagus, colorectum (in men), and female breast. The two expert reports diverge on the evidence for an association with liver cancer and colorectal cancer in women, which the IARC grades as convincing, but the WCRF/AICR as probable. Despite these discrepancies, there does, however, not seem to be any doubt, that the Population Attributable Fraction of alcohol in relation to cancer is large. As alcohol intake varies largely worldwide, so does, however, also the Population Attributable Fractions, ranging from 10% in Europe to almost 0% in countries where alcohol use is banned. Given the World Health Organization’s prediction, that alcohol intake is increasing, especially in low- and middle-income countries, and steadily high in high-income countries, the need for preventive efforts to curb the number of alcohol-related cancers seems growing, as well as the need for taking a region- and gender-specific approach in both future campaigns as well as future research. The review acknowledges the potential beneficial effects of small doses of alcohol in relation to ischaemic heart disease, but a discussion of this lies without the scope of the present study.
- In relation to planning of preventive initiatives against cancer, it is important to map the association between modifiable exposures and their effect on the risk of cancer overall, but specifically also in relation to different sub-sites. Furthermore, for risk-factors with large gender- and region-specific differences in exposure, it is preferable to build campaigns on data acquired from the specific group of interest.
- One of the main, modifiable dietary risk-factors for cancer is alcohol. Nearly 2 billion people worldwide consume alcohol regularly, and the average daily consumption has been estimated to 13 g/d approximating one drink [1]. The primary types of alcohol consumed are beer, wine and spirits—in ascending order of alcohol content [2]. Regardless of the form consumed, the International Agency for Research of Cancer (IARC) has classified its content, ethanol, in itself, as well as the first metabolite produced during metabolization in the human body; acetaldehyde, as a “group 1 carcinogen”, meaning that it has attained the highest level of evidence for a carcinogenic effect in both humans and animals [3].
- The mechanisms through which alcohol induces cancer are several. A detailed review of these are outside the scope of this review, which will instead focus on the epidemiological association between alcohol and human cancers from observational, epidemiological studies. Briefly, ethanol has a direct effect on the cells where conversion to acetaldehyde occurs; this is primarily the liver cells, where it can induce cirrhosis, but conversion also occurs in the saliva and the large intestine. Ethanol promotes production of highly reactive oxygen species, which can damage DNA, it can alter DNA methylation, and has hormonal effects, including increasing the oestradiol levels, which may affect the risk of breast cancer. Furthermore, ethanol also facilitates uptake of carcinogens from e.g., tobacco smoking in the mouth and throat, thus also increasing the risk of tobacco-induced cancers. It may also propel already existing cancers through immunosuppression, and angiogenesis, and decrease the effect of chemotherapeutics [4].
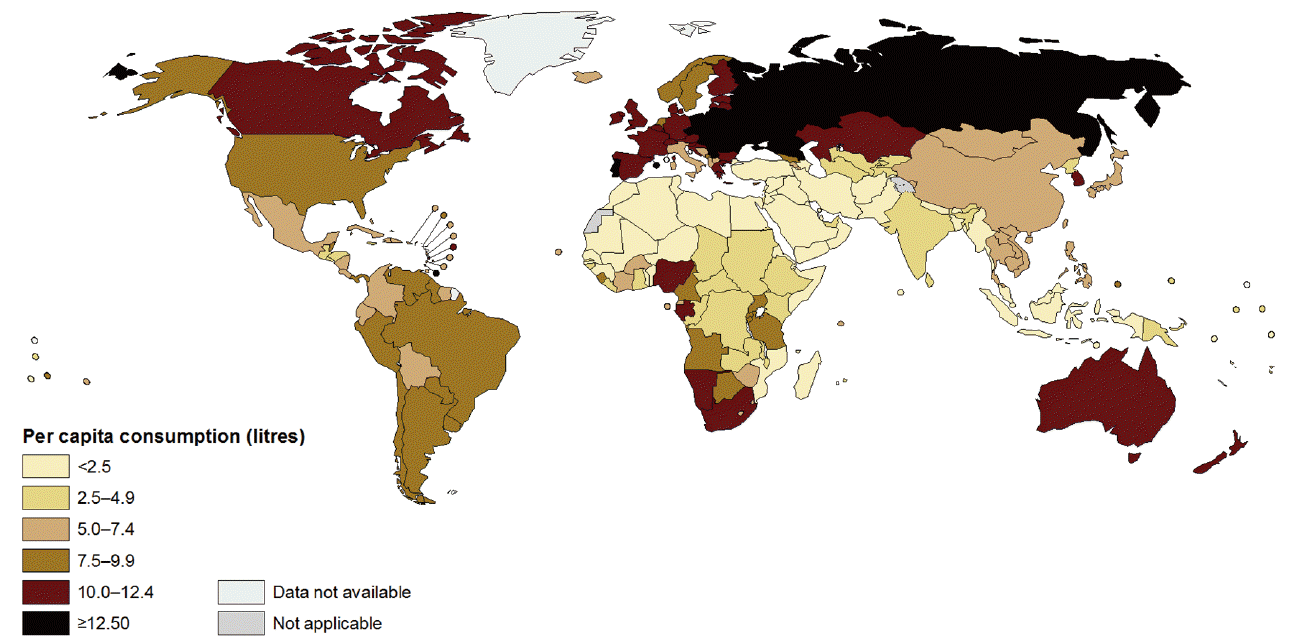
- The consumption patterns of alcohol vary to a high degree between the different parts of the world, as shown by the World Health Organization (WHO)’s Global Information System on Alcohol and Health, which showed the highest intakes (12.2 L pure alcohol/year per capita) in the Eastern European region, and the lowest in the Eastern Mediterranean region (0.7 L pure alcohol/year per capita) [5] (Figure 1) [6]. The intake of alcohol has been suggested to increase with industrialization and urbanization of a country [2].
- This review focuses on the association between alcohol consumption and the risk of those cancers, for which convincing or probable association exists, according to the IARC monograph no. 100E on alcohol and cancer [1], and the World Cancer Research Fund (WCRF)/American Institute of Cancer Research (AICR) second expert report from 2007 [2], including its continuous updates [7,8]: Oral cavity, pharynx, larynx, oesophagus, colorectum, female breast, and liver (Table 1) [1,2,8]. Following a summary and comparison of the findings of these two reports, we relate this to public health impact, with a particular focus on region-specific drinking patterns and disease tendencies.
INTRODUCTION
- The association between alcohol and human cancers have in the last decade been reviewed extensively by two large, health organizations.
- The IARC continuously publishes monographs, in which they identify environmental factors, which may increase the risk of developing cancer. Each report is written by a group of selected experts within the topic of the monograph, who review the published evidence, evaluate its weight, and classify the investigated substance as carcinogenic or not, based on evidence from both animal and human studies. The IARC has in the recent years evaluated alcohol as a potential carcinogen in 1988 [9], in 2010 [3] and most recently in a 2012 report; from which this paper will draw [1].
- The WCRF/AICR published an expert report on food, nutrition, physical activity and cancer in 2007. Here, they reviewed all relevant literature for alcohol (and other dietary factors and physical activity) and cancer and performed meta-analyses of existing data, as well as summarized their findings and published recommendations based on the evidence [2]. The project is now proceeded by a continuous update project, where the evidence is continuously updated and published on their webpage, http://www.dietandcancerreport.org/ [10]. Continuous update reports are available for the following cancers included in this paper: colorectum and breast cancer [7,8].
- Given the existence of these very extensive literature reviews and meta-analyses in the field of alcohol and cancer, the present study will summarize the findings of these two works in the following paragraphs, and compare their results. Where sound evidence exists from cohort studies, we will focus on these over case-control studies. Subsequently, we will comment on the results and relate them to public health perspectives, focusing specifically on regional patterns and consequences.
- The IARC monograph includes also studies on genetic variants and their role on the association between alcohol and cancer. This is a very relevant topic in relation to future public health initiatives, and may possibly also explain some of the regional differences seen, however, as the evidence is currently limited, it is out of the scope of the present paper to include this angl
MATERIALS
- Cancer of the Oral Cavity, Pharynx, and Larynx
- Cancers of the mouth, pharynx and larynx are the 7th most common cancer and cause of cancer death in the world. However, they show a decreasing tendency. They have a high recurrence rate, and incidence is approximately three times higher in men compared to women. The 5-year survival rate is approximately 50% [2].
- The WCRF/AICR identified 5 cohort studies examining the association between alcohol consumption and the abovementioned cancers, when conducting their study in 2007, and IARC nine studies in 2012. All studies showed an increased cancer risk for the highest intake-group compared to the lowest. The WCRF/AICR could meta-analyse two of the studies, which showed a relative risk per drink/wk of 1.24; 95% confidence interval (CI), 1.18 to 1.30 [2].
- When examining the effect of intensity and duration of alcohol intake, it has been suggested that at relatively high intakes (>60 g/d) the cancer risk is approximately three-fold that of non-drinkers, but that especially for women, the risk starts increasing already at more moderate intakes. Studies are, however, hampered by a low number of women in the highest intakegroups. With regards to effects of alcohol cessation, the time until normalization of the risk is more than 10 years [1].
- Neither the IARC nor the WCRF/AICR suggested different effects across types of alcohol consumed; e.g., beer, wine, or spirits, the IARC did, however, note that the most frequently consumed alcoholic beverage in a population tended to be most strongly related to cancer of the larynx. Both reports suggested a synergistic interaction with tobacco smoking [1,2].
- Both expert reports judge that the evidence is sufficient to conclude that consumption of alcoholic beverages is causally related to the development of cancer in the oral cavity, larynx and pharynx, and that the risk increases dose-dependently, with no lower threshold identified [1,2].
- Cancer of the Oesophagus
- Cancer of the oesophagus is the 8th most common cancer in the world, and the 6th most common cause of cancer death, as it is most often fatal. There are two common types of oesophageal cancer, squamous cell carcinoma and adenocarcinoma. Overall, the incidence of oesophageal cancer seems steady, but there has been an increase in adenocarcinomas of the oesophagus in high-income countries. The disease is twice as common in men as in women [2]. An association with alcohol primarily seem to exist for squamous cell carcinoma [1].
- Cohort studies of alcohol intake and oesophageal cancer overwhelmingly identify an increased risk in the highest intakegroups compared to the lowest [1,2]. A meta-analysis of 20 case-control studies found an effect size of 1.04 (95% CI, 1.03 to 1.05) per drink/wk. The studies presented high heterogeneity with regards to magnitude of effect, but not direction [2]. The IARC report did not include a meta-analysis, but remarked in relation to the magnitude of effect that several recent studies have consistently found a 3-8 fold increased risk with a high alcohol intake, but that an effect have also been shown at more moderate intakes; with a significant 20% increased risk per 10 g alcohol intake/d. Furthermore there seems to be a cumulative lifetime effect; with a higher risk with a longer duration of drinking, or an earlier age at alcohol debut, but also an effect of cessation with a decrease in risk beginning after 5 years, and approaching the risk of non-drinkers after 15 years [1].
- Neither the IARC nor the WCRF/AICR suggested different effects across different types of alcohol consumed, and both expert reports suggested a synergistic interaction with tobacco smoking [1,2].
- Both expert reports judge that the evidence is sufficient to conclude that consumption of alcoholic beverages is causally related to the development of oesophageal cancer, primarily squamous cell carcinoma, and that the risk increases dose-dependently, with no lower threshold identified [1,2].
- Colorectal Cancer
- Colorectal cancer is the 3rd most common cancer in the world, and the 4th most common cause of cancer death, and it is fatal in approximately half of all cases. The incidence of colorectal cancer is closely linked to industrialization and urbanization and it shows an increasing tendency in middle and low-income countries. The disease is somewhat more common in men than in women [2].
- The WCRF/AICR report identified 24 cohort studies investigating the association between alcoholic drinks and 13 on ethanol and colorectal cancer, with the vast majority showing an increased risk for the highest intake-groups [2]. In the continuous update report from 2011, a further 15 cohort studies were identified. All papers investigating alcohol as ethanol found an increased risk of colorectal cancer with intake. A meta-analysis of all papers included in the continuous update project found a 10% increased risk of colorectal and rectal cancer and an 8% increased risk of colon cancer per 10 g ethanol/d, which is a slight increase in risk compared to the numbers from the 2007 report. They also found a stronger effect for men than women with the gender-specific increased risks being 11% for men and 7% for women [8]. They ascribe this gender-difference either to the fact that men exhibit a larger spectrum of alcohol intake; allowing easier identification of an association, or that there may be hormone-related differences in alcohol metabolism or susceptibility, which affect the risk [2].
- The IARC investigated studies published after 2010 with the majority of studies showing an increased risk among high vs. low-drinkers and an effect for both colon and rectal cancer separately. They note that some studies find a weaker association for women, but in contrast to the WCRF/AICR report, they conclude that the association overall seems similar for men and women [1].
- They further comment on the dose-response relationship by citing studies finding an effect only above 20 g/d or more of intake, whereas other studies find an effect at a smaller magnitude of intake, such as 1 drink (10 g) per day. They also state that there seems to be no strong association between lifetime exposure to alcohol, frequency of drinking, and age at starting to drink [1].
- Both expert reports conclude that there does not seem to be a difference in effect with type of alcohol consumed [1,2]
- Both expert reports judge that the evidence is sufficient to conclude that consumption of alcoholic beverages is causally related to the development of colorectal cancer, and that the risk increases dose-dependently. The WCRF/AICR however graduates the judgment saying that it is convincing for men, but probable only for women [1,2].
- Female Breast Cancer
- Breast cancer is the most common cancer and the most common cause of cancer death in women, worldwide, despite being fatal in only under half of all cases. The incidence of breast cancer is, as colorectal cancer, closely linked to industrialization and urbanization. It is thus increasing rapidly in middle and low-income countries. Breast cancer is a hormone-related cancer, and the risk-factors have been found to differ between breast cancers diagnosed in premenopausal and postmenopausal women [2]. The evidence for an effect of alcohol will thus be commented on separately for these two groups, when menopausal-specific evidence exists.
- The WCRF/AICR second expert report identified 11 cohort studies examining an association between alcoholic drinks and breast cancer in 2007 [2]. The 2010 continuous update project further found 13 cohort studies on alcoholic drinks and 11 on ethanol intake. A meta-analysis of all studies in the continuous update project showed an 8% increased risk of breast cancer per 10 g ethanol/d [7]. They did not specify on premenopausal and postmenopausal breast cancer, but this was done in the second expert report, where a meta-analysis of the menopausal-specific studies showed a magnitude of effect of 1.09 (95% CI, 1.01 to 1.17) per 10 g ethanol/d for premenopausal women and 1.08 (95% CI, 1.05 to 1.10) per 10 g ethanol/d among postmenopausal women [2]. The 2010 report, however, included a meta-analysis on the association between alcohol and hormone-receptor defined breast cancers, and found that the risk of estrogen receptor positive (ER+) breast cancer increased with 12%, estrogen receptor negative (ER-) with 7%, estrogen and progesterone receptor positive (PR+) breast cancer with 11% and ER+/progesterone receptor negative (PR-) with 15% per 10 g ethanol/d. In contrast, there was no effect on breast cancers which were both estrogen and progesterone receptor negative [7].
- The IARC monograph report on 13 studies published after 2010, of which 8 showed a significant, direct association and five found no significant association [1]. There is limited evidence for an effect of frequency of drinking, age at starting alcohol consumption or cumulative lifetime intake of alcohol, and there seems to be no difference in effect with type of alcohol consumed [1].
- The IARC monograph also evaluated the association between alcohol intake and hormone-receptor specific breast cancer, which has been examined in six cohorts: four found a similar association between ER+ and ER- tumours. In contrast, two studies found positive associations for ER+ and PR+ tumours, but no association with ER-/PR- tumours, which parallels the findings of the WCRF/AICR. There is limited information on the association with triple-negative breast cancers defined by including also human epidermal growth factor receptor 2-status, as these are rather recently available data [1].
- Whereas WCRF/AICR judges that there is evidence that folate status modifies the association between alcohol and breast cancer, so that increased folate intake partially mitigates the risk induced with a high alcohol intake [2], the IARC report judges, based on studies published after 2007, that the effect of alcohol on breast cancer does not vary with folate intake [1].
- Both expert reports judge that the evidence is sufficient to conclude that consumption of alcoholic beverages is causally related to the development of breast cancer, both premenopausally and postmenopausally, and that the risk increases dose-dependently with no lower threshold identified [1,2].
- Liver Cancer
- Liver cancer is the 6th most common cancer in the world, but the third most common cause of cancer death, as it is almost always fatal. It is much more common in middle and low-income countries compared to high-income countries, with almost half of all cases occurring in China [2].
- The WCRF/AICR expert report identified 15 cohort studies examining the association between total alcoholic drinks and liver cancer; of these, 11 found an increased risk for the highest vs. the lowest intake group. Similarly, they identified 14 cohort studies examining the effect of alcohol as ethanol, of which 10 showed an increased risk among high vs. low drinkers, and a meta-analysis of these studies suggested a relative risk of 1.10 (95% CI, 1.02 to 1.17) per 10 g ethanol/day, with no heterogeneity [2].
- The association between alcohol and liver cancer should be assessed with caution, though, as a number of studies are conducted in high-risk groups, such as participants who already have cirrhosis or other liver diseases. These are often related to a high alcohol intake, but predates a liver cancer, and thus often leads to cessation or a marked reduction in alcohol intake many years before the occurrence of a liver cancer, thus confounding the association between alcohol and liver cancer [1,2]. It has repeatedly been found that most alcohol-related liver cancers develop following cirrhosis, making quantification of the risk and assessment of a threshold level difficult [11]. Both expert reports also commented, that the main risk factor for liver cancer is infection with hepatitis B or C, but that an association between alcohol and liver cancer has been shown both among people infected with hepatitis-virus as well as those not infected [1,2].
- Both expert reports judges that the evidence is sufficient to conclude that consumption of alcoholic beverages is a cause of liver cirrhosis, which predisposes to liver cancer [1,2]. The WCRF/AICR judges that consumption of alcoholic beverages is probably causally related to the development of liver cancer [2], whereas the IARC concludes that it is causally related to liver cancer [1]. The WCRF/AICR further remarks that the risk increases dose-dependently, and that no lower threshold of effect has been identified [2].
- Comments on the Evidence in Relation to Public Health in a Global Perspective
- In general, the strength of evidence for an association between alcohol and cancer risk found in the two expert reports has been strengthened compared to the previous versions [1,2,9]. Furthermore, the WCRF/AICR has been updated with a statement that no safe intake below a specific threshold exists, and that it is important to be aware that alcohol is liable to be addictive [2]. From a public health perspective, there is thus ample evidence to suggest, that the worldwide burden of cancer incidence and mortality could be brought down by decreasing alcohol consumption. Alcohol is the dietary item with the strongest and most consistent evidence for a carcinogenic effect, and its effect on global health has grown over the last decades: It was the third leading cause of global disease burden in 2010; compared to only the sixth in 1990 [12]. When considering region-specific numbers, alcohol was the leading risk-factor for disease in Eastern Europe, most of Latin America, and southern sub-Saharan Africa in 2010 [12]. Furthermore, more than 25% of the world’s alcohol consumption seems to be unrecorded [13], most likely making the numbers presented in this paper understatements of the true role of alcohol in cancer.
- Moderate alcohol consumption is, however, inversely related to ischaemic heart disease [14]. It is difficult to balance the number of deaths prevented and caused by alcohol, as this depends on the burden of cardiovascular disease vs. cancer, the age-distribution, and the drinking pattern of the population examined. The estimates will therefore vary greatly between regions. Worldwide, however, it has been estimated that the numbers of heart diseases prevented vs. caused by alcohol results in an excess of 268 000 deaths [15].
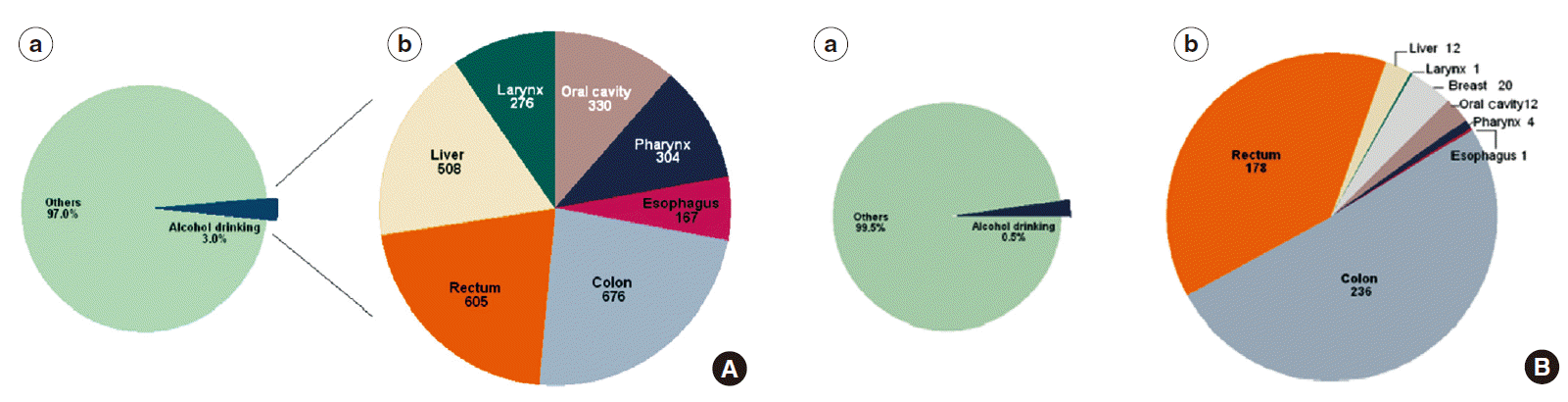
- The global burden of alcohol amounts to 3.8% of all deaths (6.3% for men and 1.1% for women) [13]. For several disease categories, the effect is stronger on mortality, compared to morbidity [14]. The proportion of cancers attributable to alcohol worldwide is 3.6% [16], and the proportion of cancer deaths is 3.5% to 5% [16,17]. However, this covers a substantial geographical variation: In Europe, the burden of cancer incidence attributable to alcohol intake has been estimated to 10% (95% CI, 7 to 13), with large variation between the subsites; the largest effect being on upper aerodigestive tract cancers [18]. In low-and middle-income countries, the cancer incidence attributable to alcohol is lower; e.g., a recent study from South Korea found a Population Attributable Fraction of 3% of incident cancers among men and 0.5% among women [19]. Figure 2 presents the Population Attributable Fraction for Korean men and women, including the number of cancer subsites ascribed to alcohol-intake [19]. The discrepancy between the relatively low population attributable fraction here, and the high consumption of alcohol in South Korea in 2010 as assessed by the WHO (Figure 1) [6], could be explained by a latency time before the effects of alcohol can be seen on cancer incidence; the proportion of teetotallers in the Korean population has been steadily declining over the last decades, suggestion that the population attributable fraction may go up in the coming years [19].
- Regardless of country, there is a large variation in the magnitude and type of alcohol-related cancers between men and women. The estimated 3.6% of cancers attributable to alcohol worldwide is unevenly split with 5.2% for men and 1.7% for women [16], reflecting the gender-specific consumption-pattern with a higher alcohol consumption and more heavy drinking occasions for men compared to women, both in high- and low-income countries [13,20]. This is exemplified by the alcohol-attributable burden of cancer in 10 of the world’s most populous countries as shown in Table 2 [13]. For all countries, the burden of cancer is larger among men compared to women. However, the difference varies greatly; being very small in Germany (9% vs. 8%), whereas in India it is 0% for women compared to 7% for men; and in Thailand it is fourfold among men compared to women. The primary alcoholrelated cancer in women is breast cancer, whereas for men it is cancer of the oral cavity, pharynx, and oesophagus [16].
- It seems that the importance of alcohol as a human carcinogen is generally underestimated at the public level; according to the WHO, the consumption is steadily high in the European and American region, with 20% of all countries showing an increasing tendency. The African countries show a more mixed tendency, but there is a worrying trend of an increasing consumption in several low- and middle-income countries in South-East Asia and the Pacific Region, most likely as a result of the economic development [13,21]. If the current increase in drinking patterns in low- and middle-income countries continues, this translates to a 3.6% increase in total cancer incidence [22]. Also in the changing drinking patterns of the lowand middle-income countries, a gender-specific effect is seen, with the increase primarily carried by men, suggesting that the future cancer burden of these countries will see a considerable increase in upper aerodigestive tract and colorectal cancers, rather than breast cancer [22]. Furthermore, several studies have shown that people with the lowest income or education are often those with the highest alcohol intake; thus contributing further to the social inequality in the burden of cancer [23-25].
- The tendency of increasing intake in the Asia/Pacific region is in contrast to the fact that most cohort studies on alcohol intake and the cancers included in this review have been conducted in the USA, Western Europe and Japan [1]. More research in developing countries is required in order to tailor public health initiatives and legislation to the specific patterns of alcohol consumption and cancer incidence of these regions. The burden of cancer is shifting to become a disease of the low-and middle-income countries also, due to the epidemiological transition with a move from infectious to lifestyle-related diseases, brought on by industrialization, an increasing standard of living, adoption of a ‘western’ lifestyle, and an increase in life expectancy [22]. In 2012, an estimated 57% of all new cancers and 65% of all cancer deaths occurred in less developed countries [26]. This is an increasing proportion since 2008 [27]. At the same time, many of the developing countries have health-care systems incapable of handling the increasing pressure of lifestyle-related diseases. Together with the fact that the advances in cancer treatment has not been as promising as the advances in treatment of other chronic diseases, this underscores the need for preventive initiatives against the main causes of cancer [17]. The types of public health initiatives required are beyond the scope of the present study, but we refer to [28,29] for a discussion of the policy implications in a global perspective.
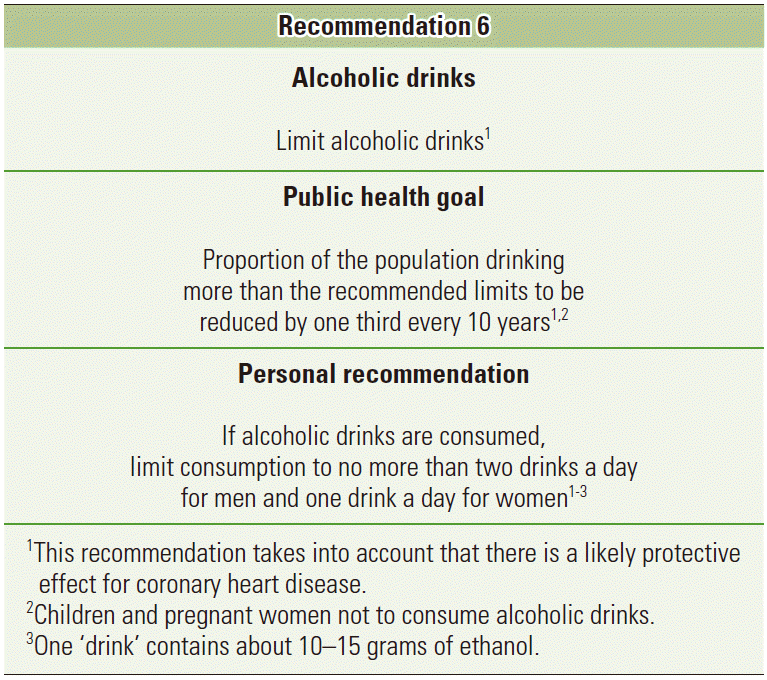
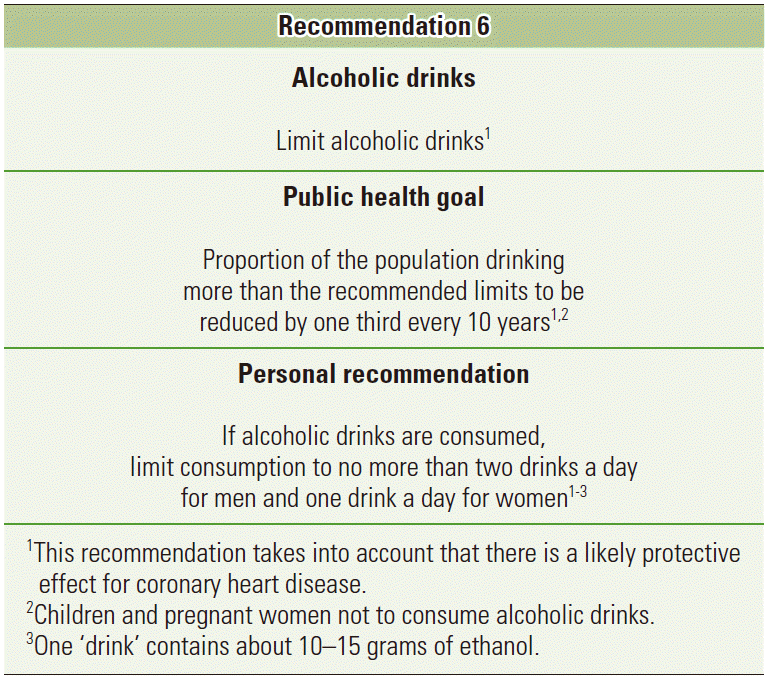
- The WCRF/AICR expert report included a specific public health recommendation. “Limit alcoholic drinks”, with the public health goal to reduce the proportion of the population drinking more than the recommended limits by one third every 10 years. They acknowledge the fact that evidence from cardiovascular epidemiology has found reduction on the risk of coronary heart disease with a modest consumption of alcohol, and thus the public health recommendation is aimed at those consuming above the recommended intakes, rather than those with a modest consumption (Figure 3) [2]. It has been shown, that a substantial proportion of cancer cases are attributable to alcohol consumption above the recommended intakes, rather than a moderate intake [18]. A worrying tendency from a public health perspective is the increasing tendency towards binge-drinking (consumption of 5+ drinks on the same occasion) in many countries, especially among the younger population [23,30,31], which is in direct disregard with the recommendations of keeping intake below the recommended limits—in many countries approximately 1 drink per day for women and 2 for men. The alcohol drinking pattern thus also needs attention in future studies [14] and public health campaigns, to make the public aware of the harmful effects of binge-drinking.
RESULTS AND DISCUSSION
- In conclusion there is strong and consistent evidence for an association between alcohol consumption and cancers of the oral cavity, pharynx, larynx, oesophagus, colorectum (in men), and breast in both expert reports. Furthermore, the IARC report claims also strong evidence for an association with colorectal cancer in women and liver cancer, whereas the WCRF/AICR grades this evidence as probable. Regardless of these minor discrepancies, public health initiatives and legislation addressing alcohol intake seems necessary to prevent cancer incidence and mortality worldwide, as alcohol contributes to approximately 3.6% of cancer incidence and 3.5% to 5% of cancer mortality. However, there is a very large variation in the Population Attributable Fraction of cancers caused by alcohol worldwide. Epidemiological studies focusing specifically on low and middle-income countries are required, as the evidence from these countries is limited compared to studies in high-income countries, and as there seems to be an increasing trend in alcohol intake here. The present review acknowledges the potential beneficial effects of small doses of alcohol in relation to ischaemic heart disease, but a discussion of this lies without the scope of the study.
CONCLUSION
ACKNOWLEDGEMENTS
-
Conflict of Interest
The authors have no conflicts of interest with the material presented in this paper.
Notes
| Exposure |
Cancer site |
||
|---|---|---|---|
| WCRF/AICR | IARC | ||
| Convincing | Alcoholic drinks | Mouth, pharynx, and larynx | Oral cavity |
| Oesophageus | Pharynx | ||
| Colorectum (men) | Larynx | ||
| Breast (premenopausal and postmenopausal) | Oesophageus | ||
| Colorectum | |||
| Breast | |||
| Liver | |||
| Probable | Liver | - | |
| Colorectum (women)1 | |||
| Limited -suggestive | - | - | |
WCRF, World Cancer Research Fund; AICR, American Institute for Cancer Research; IARC, International Agency for Cancer Research.
1 The WCRF/AICR remarks that the difference in grading of evidence in men and women is due to the lower number of studies in women, but also that alcohol seems to assert a stronger effect on colon and colorectal cancer in men compared to women.
- 1. International Agency for Research on Cancer. A review of human carcinogens. Part E: personal habits and indoor combustions. Lyon: International Agency for Research on Cancer; 2012. p. 1-575
- 2. World Cancer Research Fund; American Institute for Cancer Research. Food, nutrition, physical activity, and the prevention of cancer: a global perspective [cited 2015 Jan 5]. Available from: http://www.dietandcancerreport.org/cancer_resource_center/downloads/Second_Expert_Report_full.pdf
- 3. International Agency for Research on Cancer. Alcohol consumption and ethyl carbamate. Lyon: International Agency for Research on Cancer; 2010. p. 1-517
- 4. Seitz HK, Stickel F. Molecular mechanisms of alcohol-mediated carcinogenesis. Nat Rev Cancer 2007;7(8):599-612ArticlePubMed
- 5. World Health Organization. Global information system on alcohol and health. 2014 [cited 2014 Nov 25]. Available from: http://www.who.int/gho/alcohol/en/
- 6. World Health Organization. Total alcohol per capita (15+ year) consumption, in litres of pure alcohol. 2010 [cited 2014 Nov 25] Available from: http://gamapserver.who.int/mapLibrary/Files/Maps/Global_consumption_percapita_2010.png
- 7. World Cancer Research Fund; American Institute for Cancer Research. Breast cancer 2010 report: food, nutrition, physical activity, and the prevention of breast cancer. 2010 [cited 2015 Jan 11]. Available from: http://www.dietandcancerreport.org/cup/current_progress/breast_cancer.php
- 8. World Cancer Research Fund; American Institute for Cancer Research. Continuous update project: food, nutrition, physical activity, and the prevention of breast cancer. 2011 [cited 2015 Jan 11]. Available from: http://www.dietandcancerreport.org/cup/
- 9. International Agency for Research on Cancer. Alcohol drinking. Lyon: International Agency for Research on Cancer; 1988. p. 1-416
- 10. World Cancer Research Fund; American Institute for Cancer Research. WCRF/AIRC continuous update project 2014 [cited 2014 Nov 23]. Available from: http://www.dietandcancerreport.org/cup/index.php
- 11. Bagnardi V, Blangiardo M, La Vecchia C, Corrao G. A meta-analysis of alcohol drinking and cancer risk. Br J Cancer 2001;85(11):1700-1705ArticlePubMedPMC
- 12. Lim SS, Vos T, Flaxman AD, Danaei G, Shibuya K, Adair-Rohani H, et al. A comparative risk assessment of burden of disease and injury attributable to 67 risk factors and risk factor clusters in 21 regions, 1990-2010: a systematic analysis for the Global Burden of Disease Study 2010. Lancet 2012;380(9859):2224-2260ArticlePubMedPMC
- 13. Rehm J, Mathers C, Popova S, Thavorncharoensap M, Teerawattananon Y, Patra J. Global burden of disease and injury and economic cost attributable to alcohol use and alcoholuse disorders. Lancet 2009;373(9682):2223-2233ArticlePubMed
- 14. Rehm J, Baliunas D, Borges GL, Graham K, Irving H, Kehoe T, et al. The relation between different dimensions of alcohol consumption and burden of disease: an overview. Addiction 2010;105(5):817-843ArticlePubMedPMC
- 15. Rehm J, Room R, Monteiro M, Gmel G, Graham K, Rehn N, et al. Alcohol as a risk factor for global burden of disease. Eur Addict Res 2003;9(4):157-164ArticlePubMed
- 16. Boffetta P, Hashibe M, La Vecchia C, Zatonski W, Rehm J. The burden of cancer attributable to alcohol drinking. Int J Cancer 2006;119(4):884-887ArticlePubMed
- 17. Danaei G, Vander Hoorn S, Lopez AD, Murray CJ, Ezzati M; Comparative Risk Assessment collaborating group (Cancers). Causes of cancer in the world: comparative risk assessment of nine behavioural and environmental risk factors. Lancet 2005;366(9499):1784-1793ArticlePubMed
- 18. Schutze M, Boeing H, Pischon T, Rehm J, Kehoe T, Gmel G, et al. Alcohol attributable burden of incidence of cancer in eight European countries based on results from prospective cohort study. BMJ 2011;342: d1584ArticlePubMedPMC
- 19. Park S, Shin HR, Lee B, Shin A, Jung KW, Lee DH, et al. Attributable fraction of alcohol consumption on cancer using population-based nationwide cancer incidence and mortality data in the Republic of Korea. BMC Cancer 2014;14: 420ArticlePubMedPMC
- 20. World Cancer Research Fund. Global health atlas. 2014 [cited 2014 Nov 25]. Available from: http://apps.who.int/globalatlas/default.asp
- 21. World Health Organization. WHO Expert Committee on Problems Related to Alcohol Consumption. Geneva: World Health Organization; 2007. p. 1-57
- 22. McCormack VA, Boffetta P. Today’s lifestyles, tomorrow’s cancers: trends in lifestyle risk factors for cancer in low- and middle-income countries. Ann Oncol 2011;22(11):2349-2357ArticlePubMed
- 23. Kuntsche E, Rehm J, Gmel G. Characteristics of binge drinkers in Europe. Soc Sci Med 2004;59(1):113-127ArticlePubMed
- 24. Kim JH, Lee S, Chow J, Lau J, Tsang A, Choi J, et al. Prevalence and the factors associated with binge drinking, alcohol abuse, and alcohol dependence: a population-based study of Chinese adults in Hong Kong. Alcohol Alcohol 2008;43(3):360-370ArticlePubMed
- 25. Moller H, Tonnesen H. Alcohol drinking, social class and cancer. IARC Sci Publ 1997;(138):251-263
- 26. International Agency for Research on Cancer. GLOBOCAN 2012: estimated incidence, mortality and prevalence worldwide in 2012 [cited 2014 Nov 25]. Available from: http://globocan.iarc.fr/Pages/fact_sheets_cancer.aspx
- 27. Ferlay J, Shin HR, Bray F, Forman D, Mathers C, Parkin DM. Estimates of worldwide burden of cancer in 2008: GLOBOCAN 2008. Int J Cancer 2010;127(12):2893-2917ArticlePubMed
- 28. Anderson P, Chisholm D, Fuhr DC. Effectiveness and cost-effectiveness of policies and programmes to reduce the harm caused by alcohol. Lancet 2009;373(9682):2234-2246ArticlePubMed
- 29. Casswell S, Thamarangsi T. Reducing harm from alcohol: call to action. Lancet 2009;373(9682):2247-2257ArticlePubMed
- 30. Assanangkornchai S, Mukthong A, Intanont T. Prevalence and patterns of alcohol consumption and health-risk behaviors among high school students in Thailand. Alcohol Clin Exp Res 2009;33(12):2037-2046ArticlePubMed
- 31. Miller JW, Naimi TS, Brewer RD, Jones SE. Binge drinking and associated health risk behaviors among high school students. Pediatrics 2007;119(1):76-85ArticlePubMed
REFERENCES
Figure & Data
References
Citations

- Alcohol and Periodontal Disease: A Narrative Review
Utsav H Gandhi, Amit Benjamin, Shreya Gajjar, Tanvi Hirani, Khushboo Desai, Bansariben B Suhagia, Rahnuma Ahmad, Susmita Sinha, Mainul Haque, Santosh Kumar
Cureus.2024;[Epub] CrossRef - Biosynthesis of anticancer phytochemical compounds and their chemistry
Amandeep Dogra, Jitender Kumar
Frontiers in Pharmacology.2023;[Epub] CrossRef - Association of Healthy Diet and Physical Activity With Breast Cancer: Lifestyle Interventions and Oncology Education
Tiantian Jia, Yufeng Liu, Yuanyuan Fan, Lintao Wang, Enshe Jiang
Frontiers in Public Health.2022;[Epub] CrossRef - Respiratory Tract Cancer Incidences across Industry Groups: A Nationwide Cohort Study with More Than 70 Million Person-Years of Follow-Up
Seong-Uk Baek, Woo-Ri Lee, Ki-Bong Yoo, Jun-Hyeok Choi, Kyung-Eun Lee, Wanhyung Lee, Jin-Ha Yoon
Cancers.2022; 14(21): 5219. CrossRef - Cancer Incidence and Risk of Multiple Cancers after Environmental Asbestos Exposure in Childhood—A Long-Term Register-Based Cohort Study
Sofie Bünemann Dalsgaard, Else Toft Würtz, Johnni Hansen, Oluf Dimitri Røe, Øyvind Omland
International Journal of Environmental Research and Public Health.2021; 19(1): 268. CrossRef - Combining population projections with quasi-likelihood models: A new way to predict cancer incidence and cancer mortality in Austria up to 2030
Johannes Klotz, Monika Hackl, Markus Schwab, Alexander Hanika, Daniela Haluza
Demographic Research.2019; 40: 503. CrossRef - Psychosocial Motivators for Moderate Drinking among Young Asian Flushers in Singapore
Hye Kyung Kim, Rachel Lim Si En, Dorothy Wong Kang Min
International Journal of Environmental Research and Public Health.2019; 16(11): 1897. CrossRef - My own personal hell: approaching and exceeding thresholds of too much alcohol
Mark Burgess, Richard Cooke, Emma L. Davies
Psychology & Health.2019; 34(12): 1451. CrossRef - Nutrition and Breast Cancer: A Literature Review on Prevention, Treatment and Recurrence
Paola De Cicco, Maria Valeria Catani, Valeria Gasperi, Matteo Sibilano, Maria Quaglietta, Isabella Savini
Nutrients.2019; 11(7): 1514. CrossRef - The Effect of Okra (Abelmoschus esculentus (L.) Moench) Seed Extract on Human Cancer Cell Lines Delivered in Its Native Form and Loaded in Polymeric Micelles
Watcharaphong Chaemsawang, Weerapong Prasongchean, Konstantinos I. Papadopoulos, Garnpimol Ritthidej, Suchada Sukrong, Phanphen Wattanaarsakit
International Journal of Biomaterials.2019; 2019: 1. CrossRef - Colorectal Cancer and Alcohol Consumption—Populations to Molecules
Marco Rossi, Ahmad Usman, Ali Keshavarzian, Faraz Bishehsari
Cancers.2018; 10(2): 38. CrossRef - Differential Expression of Prostaglandin I2 Synthase Associated with Arachidonic Acid Pathway in the Oral Squamous Cell Carcinoma
Anelise Russo, Patrícia M. Biselli-Chicote, Rosa S. Kawasaki-Oyama, Márcia M. U. Castanhole-Nunes, José V. Maniglia, Dalísio de Santi Neto, Érika C. Pavarino, Eny M. Goloni-Bertollo
Journal of Oncology.2018; 2018: 1. CrossRef
 KSPM
KSPM

 PubReader
PubReader ePub Link
ePub Link Cite
Cite