Articles
- Page Path
- HOME > J Prev Med Public Health > Volume 55(5); 2022 > Article
-
Special Article
Statin Intake and Gastric Cancer Risk: An Updated Subgroup Meta-analysis Considering Immortal Time Bias -
Jong-Myon Bae

-
Journal of Preventive Medicine and Public Health 2022;55(5):424-427.
DOI: https://doi.org/10.3961/jpmph.22.209
Published online: August 18, 2022
Department of Preventive Medicine, Jeju National University College of Medicine, Jeju, Korea
- Corresponding author: Jong-Myon Bae, Department of Preventive Medicine, Jeju National University College of Medicine, 102 Jejudaehak-ro, Jeju 63243, Korea, E-mail: jmbae@jejunu.ac.kr
• Received: May 9, 2022 • Accepted: August 11, 2022
Copyright © 2022 The Korean Society for Preventive Medicine
This is an Open Access article distributed under the terms of the Creative Commons Attribution Non-Commercial License (https://creativecommons.org/licenses/by-nc/4.0/) which permits unrestricted non-commercial use, distribution, and reproduction in any medium, provided the original work is properly cited.
ABSTRACT
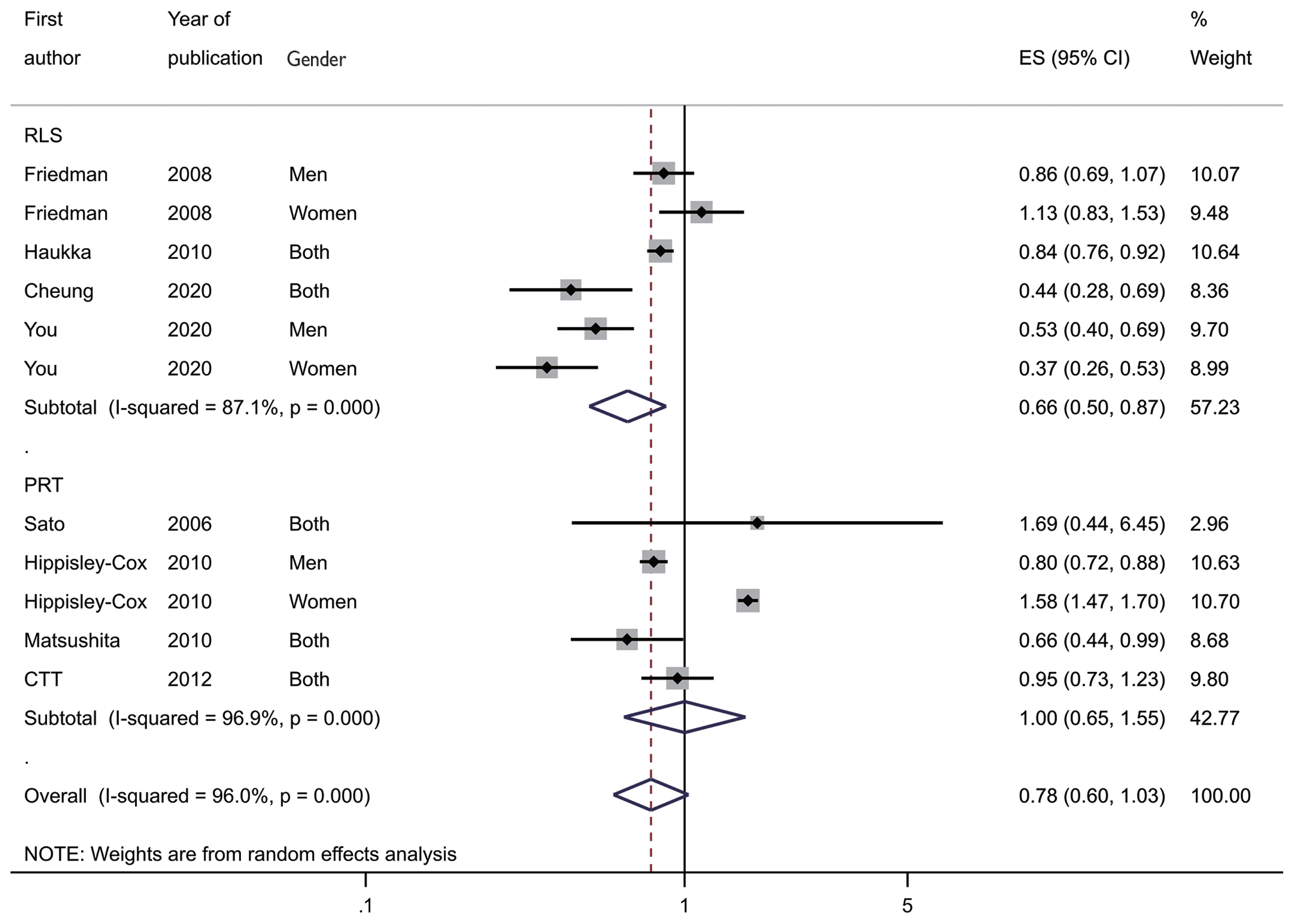
- A retrospective record-linkage study (RLS) based on medical records containing drug prescription histories involves immortal time bias (ITB). Thus, it is necessary to control for this bias in the research planning and analysis stages. Furthermore, a summary of a meta-analysis including RLSs that did not control for ITB showed that specific drugs had a preventive effect on the occurrence of the disease. Previous meta-analytic results of three systematic reviews evaluating the association between statin intake and gastric cancer risk showed that the summary hazard ratio (sHR) of the RLSs was lower than 1 and was statistically significant. We should consider the possibility of ITB in the sHR of RLSs and interpret the results carefully.
- This study was waived by an ethics review board because the study subjects were published articles.
Ethics Statement
-
CONFLICT OF INTEREST
The author has no conflicts of interest associated with the material presented in this paper.
-
FUNDING
This work was supported by the 2022 education, research, and student guidance grant funded by Jeju National University.
Notes
ACKNOWLEDGEMENTS
Figure 1Forest plot by study design. RLS, record-linkage study; PRT, post-hoc analysis of a randomized trial; ES, effect size ; CI, confidence interval.


Table 1Summary hazard ratios (sHRs) and their confidence intervals (CIs) of the published systematic reviews
| Study | Searching | Selected | sHR (95% CI) | I-squared (%) |
|---|---|---|---|---|
| Singh et al. 2013 [10] | Dec 2012 | 3 PRT | 0.83 (0.66, 1.05) | - |
|
|
||||
| Wu et al. 2013 [11] | Mar 2013 | 3 PRT | 0.73 (0.53, 0.93) | 28.5 |
| 3 RLS | 0.87 (0.77, 0.99) | 24.7 | ||
|
|
||||
| Seo et al. 2022 [12] | 2020 | 5 RLS | 0.71 (0.59, 0.85) | 68.0 |
- 1. Park BJ, Cho YK, Kim SA. Construction of the Korea Elderly Pharmacoepidemiologic Cohort: drug utilization review of cephalosporins in geriatric inpatients. Pharmacoepidemiol Drug Saf 2001;10(6):487-492ArticlePubMed
- 2. Jones JK. Pharmacogenetics and pharmacoepidemiology. Pharmacoepidemiol Drug Saf 2001;10(5):457-461ArticlePubMed
- 3. Suissa S, Dell’Aniello S. Time-related biases in pharmacoepidemiology. Pharmacoepidemiol Drug Saf 2020;29(9):1101-1110ArticlePubMedPDF
- 4. Suissa S. Immortal time bias in observational studies of drug effects. Pharmacoepidemiol Drug Saf 2007;16(3):241-249ArticlePubMed
- 5. Suissa S. Immortal time bias in pharmaco-epidemiology. Am J Epidemiol 2008;167(4):492-499ArticlePubMed
- 6. Machlowska J, Baj J, Sitarz M, Maciejewski R, Sitarz R. Gastric cancer: epidemiology, risk factors, classification, genomic characteristics and treatment strategies. Int J Mol Sci 2020;21(11):4012ArticlePubMedPMC
- 7. Shah SC, Peek RM Jr. Chemoprevention against gastric cancer. Gastrointest Endosc Clin N Am 2021;31(3):519-542ArticlePubMedPMC
- 8. Liu Q, Xia H, Zhou S, Tang Q, Zhou J, Ren M, et al. Simvastatin inhibits the malignant behaviors of gastric cancer cells by simultaneously suppressing YAP and β-catenin signaling. Onco Targets Ther 2020;13: 2057-2066PubMedPMC
- 9. den Hoed CM, Kuipers EJ. Gastric cancer: how can we reduce the incidence of this disease? Curr Gastroenterol Rep 2016;18(7):34ArticlePubMedPMCPDF
- 10. Singh PP, Singh S. Statins are associated with reduced risk of gastric cancer: a systematic review and meta-analysis. Ann Oncol 2013;24(7):1721-1730ArticlePubMed
- 11. Wu XD, Zeng K, Xue FQ, Chen JH, Chen YQ. Statins are associated with reduced risk of gastric cancer: a meta-analysis. Eur J Clin Pharmacol 2013;69(10):1855-1860ArticlePubMedPDF
- 12. Seo SI, Park CH, Kim TJ, Bang CS, Kim JY, Lee KJ, et al. Aspirin, metformin, and statin use on the risk of gastric cancer: a nationwide population-based cohort study in Korea with systematic review and meta-analysis. Cancer Med 2022;11(4):1217-1231ArticlePubMedPDF
- 13. Harris RJ, Deeks JJ, Altman DG, Bradburn MJ, Harbord RM, Sterne JA. Metan: fixed-and random-effects meta-analysis. Stata J 2008;8(1):3-28ArticlePDF
- 14. Bae JM. Meta-epidemiology. Epidemiol Health 2014;36: e2014019ArticlePubMedPMC
- 15. Friedman GD, Flick ED, Udaltsova N, Chan J, Quesenberry CP Jr, Habel LA. Screening statins for possible carcinogenic risk: up to 9 years of follow-up of 361,859 recipients. Pharmacoepidemiol Drug Saf 2008;17(1):27-36ArticlePubMed
- 16. Haukka J, Sankila R, Klaukka T, Lonnqvist J, Niskanen L, Tanskanen A, et al. Incidence of cancer and statin usage--record linkage study. Int J Cancer 2010;126(1):279-284ArticlePubMed
- 17. Cheung KS, Chan EW, Wong AY, Chen L, Seto WK, Wong IC, et al. Statins were associated with a reduced gastric cancer risk in patients with eradicated helicobacter pylori infection: a territory-wide propensity score matched study. Cancer Epidemiol Biomarkers Prev 2020;29(2):493-499ArticlePubMedPDF
- 18. You HS, You N, Lee JW, Lim HJ, Kim J, Kang HT. Inverse association between statin use and stomach cancer incidence in individuals with hypercholesterolemia, from the 2002–2015 NHIS-HEALS data. Int J Environ Res Public Health 2020;17(3):1054ArticlePubMedPMC
- 19. Sato S, Ajiki W, Kobayashi T, Awata N; PCS Study Group. Pravastatin use and the five-year incidence of cancer in coronary heart disease patients: from the prevention of coronary sclerosis study. J Epidemiol 2006;16(5):201-206ArticlePubMedPMC
- 20. Hippisley-Cox J, Coupland C. Unintended effects of statins in men and women in England and Wales: population based cohort study using the QResearch database. BMJ 2010;340: c2197ArticlePubMedPMC
- 21. Matsushita Y, Sugihara M, Kaburagi J, Ozawa M, Iwashita M, Yoshida S, et al. Pravastatin use and cancer risk: a meta-analysis of individual patient data from long-term prospective controlled trials in Japan. Pharmacoepidemiol Drug Saf 2010;19(2):196-202ArticlePubMed
- 22. Cholesterol Treatment Trialists’ (CTT) Collaboration, Emberson JR, Kearney PM, Blackwell L, Newman C, Reith C, et al. Lack of effect of lowering LDL cholesterol on cancer: meta-analysis of individual data from 175,000 people in 27 randomised trials of statin therapy. PLoS One 2012;7(1):e29849ArticlePubMedPMC
- 23. Oh TK, Song IA. Drug-specific and dosage effects of statins and the risk of cancer: a population-based cohort study in South Korea. Eur J Cancer Prev 2021;30(2):188-194ArticlePubMed
- 24. Cho MH, Yoo TG, Jeong SM, Shin DW. Association of aspirin, metformin, and statin use with gastric cancer incidence and mortality: a nationwide cohort study. Cancer Prev Res (Phila) 2021;14(1):95-104ArticlePubMedPDF
- 25. Shariff SZ, Cuerden MS, Jain AK, Garg AX. The secret of immortal time bias in epidemiologic studies. J Am Soc Nephrol 2008;19(5):841-843ArticlePubMed
REFERENCES
Figure & Data
References
Citations
Citations to this article as recorded by 

- Sodium-glucose cotransporter-2 inhibitors use and the risk of gout: a systematic review and meta-analysis
Shih-Wei Lai, Bing-Fang Hwang, Yu-Hung Kuo, Chiu-Shong Liu, Kuan-Fu Liao
Frontiers in Endocrinology.2023;[Epub] CrossRef
 KSPM
KSPM

 PubReader
PubReader ePub Link
ePub Link Cite
Cite