Articles
- Page Path
- HOME > J Prev Med Public Health > Volume 56(5); 2023 > Article
-
Original Article
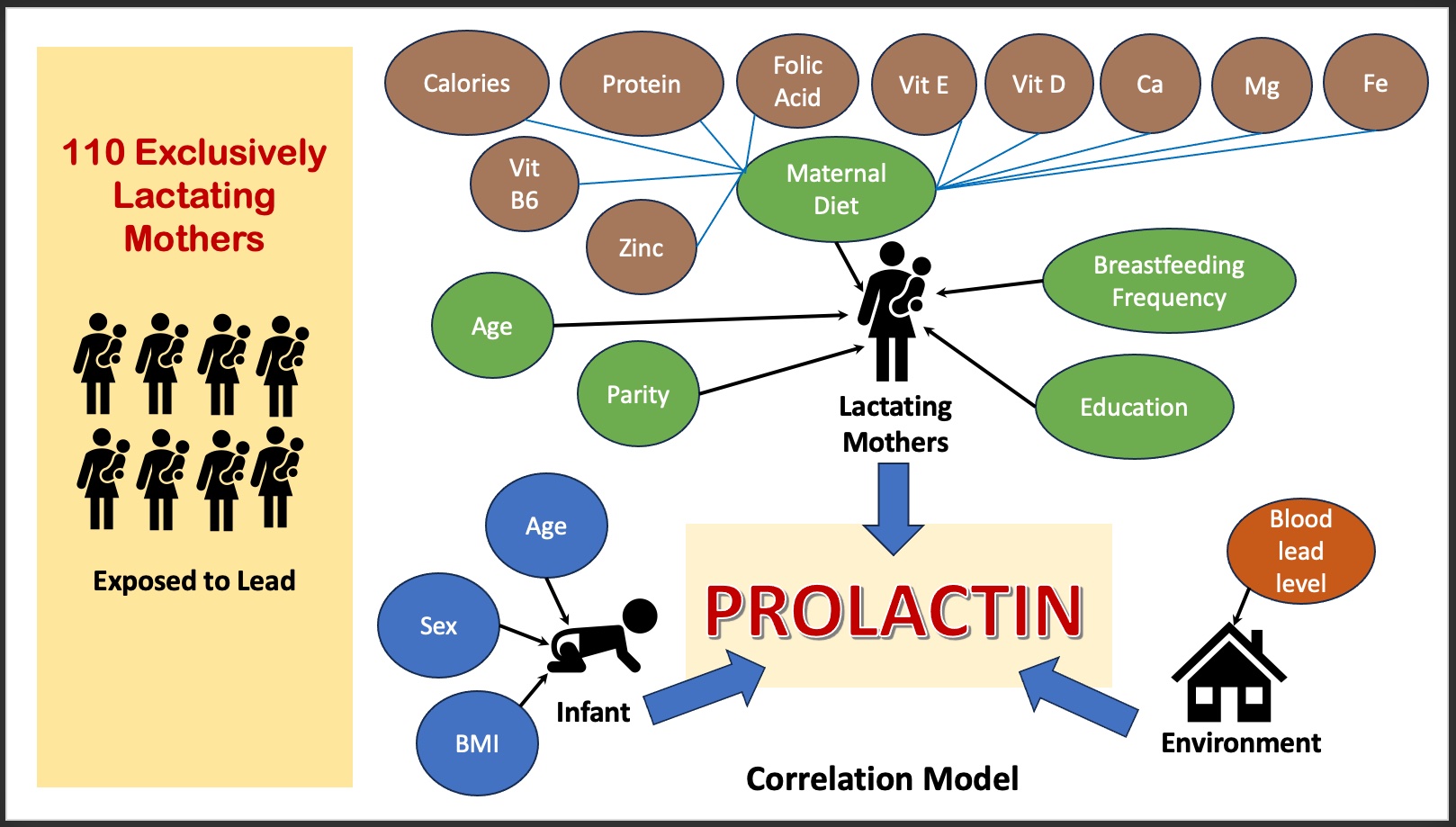
Correlations Among Maternal and Infant Factors, Lead Exposure, and Serum Prolactin Levels During Lactation: A Cross-sectional Study in Indonesia -
Linda Ratna Wati1,2
 , Djanggan Sargowo3
, Djanggan Sargowo3 , Tatit Nurseta4
, Tatit Nurseta4 , Lilik Zuhriyah5
, Lilik Zuhriyah5 , Bambang Rahardjo4
, Bambang Rahardjo4
-
Journal of Preventive Medicine and Public Health 2023;56(5):422-430.
DOI: https://doi.org/10.3961/jpmph.23.238
Published online: August 22, 2023
- 1,362 Views
- 141 Download
1Doctoral Study Program in Medical Science, Faculty of Medicine, Universitas Brawijaya, Malang, Indonesia
2Department of Midwifery, Faculty of Medicine, Universitas Brawijaya, Malang, Indonesia
3Department of Cardiology and Vascular Medicine, Faculty of Medicine, Universitas Brawijaya/Universitas Brawijaya Hospital, Malang, Indonesia
4Department of Obstetrics and Gynaecology, Faculty of Medicine, Universitas Brawijaya, Malang, Indonesia
5Department of Public Health, Faculty of Medicine, Universitas Brawijaya, Malang, Indonesia
- Corresponding author: Tatit Nurseta, Department of Obstetrics and Gynaecology, Faculty of Medicine, Universitas Brawijaya, Jl. Veteran No.10-11, Ketawanggede, Malang 65145, Indonesia, E-mail: tns_obg.fk@ub.ac.id
Copyright © 2023 The Korean Society for Preventive Medicine
This is an Open Access article distributed under the terms of the Creative Commons Attribution Non-Commercial License (https://creativecommons.org/licenses/by-nc/4.0/) which permits unrestricted non-commercial use, distribution, and reproduction in any medium, provided the original work is properly cited.
ABSTRACT
-
Objectives
- Prolactin is vital for breastfeeding and milk production, and its secretion is influenced by factors related to the mother, infant, and environment. To date, no study has concurrently investigated the correlation of these factors with serum prolactin levels during lactation. Therefore, the objective of this study was to investigate the correlations among maternal and infant factors, lead exposure, and serum prolactin levels during lactation.
-
Methods
- A cross-sectional approach was employed in Surabaya, Indonesia, among 110 exclusively lactating mothers. The mothers’ daily diets were determined using multiple 24-hour recalls, while blood lead levels were measured with inductively coupled plasma mass spectrometry. Serum prolactin levels were assessed using the electrochemiluminescence immunoassay. For bivariate analysis, we employed the Spearman correlation, Mann-Whitney, and Kruskal-Wallis tests, while for multivariate analysis, we utilized multiple linear regression.
-
Results
- The average serum prolactin level of the lactating mothers was 129.19±88.96 ng/mL. Positive correlations were found between serum prolactin levels and breastfeeding frequency (p<0.001), protein intake (p<0.001), and calcium intake (p=0.011) but had negative correlation with blood lead levels (p<0.001) and vitamin B6 intake (p=0.003). Additionally, prolactin levels were not significantly associated with maternal age; parity; intake of calories, vitamin D, vitamin E, zinc, folic acid, magnesium, or iron; infant age; or infant sex.
-
Conclusions
- Breastfeeding frequency had a stronger positive relationship with serum prolactin levels than protein and calcium intake. However, lead exposure was associated with reduced serum prolactin levels during lactation. Consequently, specific interventions from policymakers are necessary to manage breastfeeding in mothers exposed to lead.
- Breastfeeding is important for the nutrition and well-being of a newborn. The World Health Organization (WHO) recommends that infants be exclusively breastfed for the first 6 months of life, with continued breastfeeding for up to 2 years [1]. Prolactin is a vital hormone in breastfeeding and milk production, with insufficient levels often leading to difficulties in breastfeeding [2]. Prolactin promotes the growth of the mammary glands [3] and stimulates the alveolar epithelial cells of the breast to synthesize milk components, including lactose, casein, and lipids [4].
- The level of prolactin is dynamic and can fluctuate based on a variety of factors related to the mother, infant, environment, and physiology [5,6]. Several studies have extensively discussed maternal and infant factors that can disrupt the maintenance of prolactin levels, including parity, maternal age, maternal protein intake, breastfeeding frequency, and infant age [7,8]. However, the findings of these studies are inconsistent. Moreover, no studies have yet explored these characteristics in conjunction with environmental factors, such as exposure to lead.
- Lead pollution continues to be a key concern in Indonesia, where air lead concentrations vary from 0.2 ng/m3 to 2664.2 ng/m3. The city of Surabaya is reported to have the highest concentration, at 2664.2 ng/m3 [9]. The WHO air quality guidelines stipulate a safe limit for airborne lead of 0.5 μg/m3 [10]. The average lead content in Indonesia’s seawater is 0.055 ppm, while sediment contains approximately 13.59 mg/kg [11]. Surabaya, a major population center, has a dangerously high lead level, with the average content in both air and water surpassing the Environmental Ministry’s standard of 0.008 μg/mL [12]. Furthermore, shellfish from Surabaya’s eastern coast have been found to contain heavy metals in amounts that exceed published standards [13].
- Lead is a toxic metal that can have detrimental effects on various organs, with the potential to disrupt the endocrine system [14]. It impacts female reproductive health, influencing menstruation, fertility, the timing of conception, hormone production, blood circulation, and pregnancy [15]. When accumulated in the brain, lead can impair neuroendocrine function, leading to adverse effects on the dopaminergic system and associated behavioral abnormalities [16]. According to guidelines from the Centers for Disease Control and Prevention (CDC), mothers with blood lead levels (BLLs) exceeding 5 μg/dL should closely monitor these levels to ensure safe breastfeeding. Furthermore, mothers with BLLs over 40 μg/dL are strongly advised against breastfeeding [15]. Research indicates that pregnant female exposed to lead may experience a decrease in prolactin levels [17]. However, few studies have investigated the specific effects of lead exposure during the lactation period.
- The purpose of this study was to investigate the correlations of maternal factors, infant factors, and lead exposure with serum prolactin levels in breastfeeding mothers living in the coastal region of Surabaya, Indonesia.
INTRODUCTION
- Participants
- The research was conducted at 2 community health centers located in the coastal area of Surabaya: Benowo Health Center and Kenjeran Health Center. The study population consisted of mothers who were exclusively breastfeeding and who met the following inclusion criteria: had an infant between 1 month and 6 months old, did not consume any breast milk boosters containing dopamine antagonists, had resided in the study area for at least 6 months, were not under particular stress or smokers, and had a full-term infant who was breastfed at least 8 times per day. Mothers with pre-existing medical conditions such as thyroid disease or diabetes mellitus, as well as those with twins, were excluded from the study. The sample size was determined to be 110 participants, as calculated using the Lemeshow formula [18]. This was based on the proportion of mothers exclusively breastfeeding in the study area 6 months prior, which was 7.6%. A total of 108 participants were deemed necessary, with an additional 20% included to account for potential dropouts. However, 14 participants were ultimately excluded based on the predefined exclusion criteria, and 7 withdrew during the course of the study. The participants were chosen using a stratified random sampling technique, considering their socioeconomic status. Individuals from both low and middle socioeconomic groups were randomly selected, ensuring that both groups had a comparable risk of lead exposure.
- Maternal and Infant Characteristics
- Maternal factors, such as age, parity, diet, and breastfeeding frequency, along with infant factors like the infant’s age and sex, were determined through interviews. Breastfeeding frequency was calculated as the average number of times breastfeeding had occurred per day over the 3 days prior to the collection of the mother’s blood sample. Maternal diet, including the intake of protein, calories, vitamin B6, vitamin D, vitamin E, zinc, folic acid, calcium, magnesium, and iron, was assessed using multiple 24-hour food recalls. Three dietary evaluations were conducted: 2 on weekdays and 1 on the weekend. These assessments were performed 1 week prior to the collection of maternal blood samples. The quantification of maternal diet was performed using Nutrisurvey software, based on the “Indonesian Food Composition” table published by the Ministry of Health [19].
- Collection and Measurement of Blood Samples
- Twenty-milliliter blood samples were collected between 08:00 a.m. and 10:00 a.m., 30 minutes after breastfeeding, for the analysis of both prolactin levels and BLL. These samples were collected in labeled vacutainers containing ethylenediaminetetraacetic acid and were analyzed at Prodia Clinical Laboratory within 1 hour of collection. The blood serum was then appropriately stored at −20°C until further use. BLL was measured using inductively coupled plasma mass spectrometry (ICP-MS; Model 7700, Agilent Technologies Inc., Santa Clara, CA, USA) with a detection limit of 0.09 ng/L [20]. Serum prolactin levels were also determined using the Elecsys® Prolactin II immunoassay, a sandwich electrochemiluminescence technique provided by Roche Diagnostics (Roche cobas e 411; Mannheim, Germany) [21]. Both BLLs and serum prolactin levels were analyzed at Prodia Clinical Laboratory, which holds accreditation from the College of American Pathologists, as well as ISO 15189 certification.
- Statistical Analysis
- Statistical analysis was conducted using SPSS version 26 (IBM Corp., Armonk, NY, USA). The Spearman correlation was employed to examine the bivariate relationship between maternal and infant factors, BLL, and serum prolactin levels. The Mann-Whitney and Kruskal-Wallis tests were utilized to analyze differences in maternal and infant factors based on BLL and prolactin levels. The stepwise method was employed to derive the optimal multiple linear regression model. In this analysis, only variables with a significance level of p<0.20 in the bivariate analysis were selected [6]. A p-value of less than 0.05 was considered to indicate statistical significance.
- Ethics Statement
- The study protocol was approved by the Health Research Ethics Commission at the Faculty of Medicine of Universitas Brawijaya (No. 366/EC/KEPK-S3/12/2021).
METHODS
- Maternal and Infant Characteristics
- Of the 110 participating mothers, 67.3% were between 20 years and 30 years old. Furthermore, 65.4% had given birth on multiple times, 70.9% had a low level of education, 92.7% breastfed their infants between 8 times and 17 times daily, and 57.3% had resided in the study area for more than a decade. Regarding infant characteristics, 55.4% were male, 61.8% were between 1 month and 3 months old, and 65.5% fell within the normal weight range for their age, as shown in Table 1.
- Correlations of Maternal and Infant Factors with Maternal Blood Lead Levels
- The assessment of maternal diet revealed that 55 respondents (50.9%) had a high protein intake, at more than 80 g/day. The participants’ intake of animal protein (45.47±20.10 g/day) was higher than that of plant-based protein (37.59±16.45 g/day). Furthermore, 88 participants, or 81.5%, had an adequate total calorie intake. The mean typical daily vitamin and mineral consumption of the mothers included 1.51±0.76 mg/day of vitamin B6, 4.51±1.76 μg/day of vitamin D, 5.40±2.11 mg/day of vitamin E, 9.96±3.77 mg/day of zinc, 165.27±48.74 μg/day of folic acid, 940.99±270.98 mg/day of calcium, 327.82± 94.02 mg/day of magnesium, and 8.23±2.34 mg/day of iron. The maternal examination indicated that the average BLL was 3.64±1.38 μg/dL. Among the participants, 89 had BLLs of less than 5 μg/dL, while 21 had levels that necessitated intensive monitoring, as they were above 5 μg/dL. The results of the data analysis, as shown in Table 2, revealed differences in BLL in relation to maternal protein intake (p=0.019), calcium intake (p<0.001), and infant sex (p=0.009).
- Correlations of Maternal Factors, Infant Factors, and Maternal Blood Lead Levels With Serum Prolactin Levels
- The average serum prolactin level was 129.19±88.96 ng/mL, with a range of 13.1 ng/mL to 345.9 ng/mL. The Kolmogorov–Smirnov test for normality revealed that only the data for calorie and calcium intake followed a normal distribution (p>0.05). The results of data analysis using the Mann-Whitney and Kruskal-Wallis tests revealed significant differences in serum prolactin levels in relation to breastfeeding frequency (p<0.001), protein intake (p<0.001), calcium intake (p=0.021), and infant age (p=0.006). These findings are detailed in Table 2.
- Furthermore, Spearman correlations revealed significant relationships between serum prolactin level and breastfeeding frequency (r=0.780, p<0.001), protein intake (r=0.511, p<0.001), calcium intake (r=0.501, p<0.001), and calorie intake (r=0.275, p=0.004). Prolactin level was found to decrease with vitamin B6 intake (r=−0.459, p<0.001), infant age (r=−0.387, p<0.001), zinc intake (r=−0.278, p=0.003), and BLL (r=−0.299, p<0.001). However, no significant association was found between prolactin level and maternal age (r=−0.023, p=0.814); parity (r= −0.093, p=0.335); vitamin D (r=−0.172, p=0.073), vitamin E (r=0.179, p=0.061), iron (r=−0.019, p=0.841), folic acid (r=0.092, p=0.337), or magnesium intake (r=0.117, p=0.222); or the sex of the infant (r=−0.137, p=0.154).
- Multiple linear regression analysis yielded a statistically significant adjusted final model based on the stepwise method (p<0.001, R2=0.775) (Table 3). This model indicated that 77.5% of the factors included in the model influenced serum prolactin levels during lactation. Positive correlations were observed between serum prolactin level and breastfeeding frequency (r=0.677), protein intake (r=0.218), and calcium intake (r=0.139), while BLL (r=−0.323) and vitamin B6 intake (r=−0.169) were negatively correlated with prolactin level.
RESULTS
- To our knowledge, this is the first study to concurrently examine the influence of maternal, infant, and environmental factors on serum prolactin levels among lactating mothers. The impact of lead exposure on serum prolactin levels was a novel finding of the study. The multivariate analysis indicated that serum prolactin levels were correlated with breastfeeding frequency; protein, calcium, and vitamin B6 intake; and BLL.
- In this study, the factor most strongly correlated with serum prolactin level was breastfeeding frequency. Of the participants, 92.7% breastfed between 8 times and 17 times per day. This observation is consistent with the findings of Huang and Chih [22], who noted an association between increased prolactin levels and a breastfeeding frequency of more than 10 times per day. The act of an infant sucking impacts the paraventricular nucleus, which plays a role in prolactin release. Additionally, infant sucking can suppress the secretion of dopamine, a hormone known to inhibit prolactin. To keep dopamine production levels low, continued breastfeeding is necessary [7]. The total amount of prolactin secreted daily depends on both the frequency and duration of breastfeeding [23].
- In addition to breastfeeding frequency, certain elements of a mother’s diet, including protein, calcium, and vitamin B6 intake, were found to correlate with serum prolactin levels. Food consumption plays a crucial role in the synthesis of prolactin. During lactation, it is recommended to consume an additional 25 g/day of protein as part of the daily allowance [24]. This study demonstrated a positive correlation between protein intake and serum prolactin level. The consumption of protein is vital, as it provides the necessary amino acids for the synthesis of the prolactin hormone. Eating protein-rich foods significantly stimulates prolactin secretion [25]. This study also indicated a correlation between calcium intake and serum prolactin levels. Breastfeeding mothers with a higher calcium intake exhibited higher serum prolactin levels than those with a lower calcium intake. This is likely due to the role of calcium in lowering BLL, as suggested by research conducted by Syofyan et al. [26]. Their study revealed a decrease in BLL in children who were given 800 mg/day of calcium supplements for 3 months. In contrast, the intake of vitamin B6 was found to negatively correlate with serum prolactin level. This finding is supported by research conducted by Zhuo et al. [27], which demonstrated a decrease in prolactin levels of up to 68.1% after participants were administered a vitamin B6 treatment (300 mg every 12 hours for 16 weeks). However, the correlation between serum prolactin levels and maternal caloric intake was found to be insignificant. While the synthesis of prolactin requires adequate energy intake, the same caloric intake does not necessarily yield the same energy output, as this depends on the proportion of protein, fat, and carbohydrates consumed [28].
- This study revealed that all breastfeeding mothers within the study population exhibited detectable BLLs, with a mean concentration of 3.64 μg/dL. Moreover, 19.09% of these mothers had BLLs above the safe thresholds. These results may be due to the numerous sources of lead exposure and its extended elimination phase [29]. Furthermore, the mobilization of lead from bone during lactation is more pronounced than during pregnancy, likely due to insufficient dietary calcium intake [30]. The average maternal calcium intake in this study was 940.99 mg/day, which is below the recommended daily intake for breastfeeding mothers. Research carried out between 2000 and 2015 in various countries, as cited by Rebelo and Caldas [31], revealed higher levels of lead in breast milk compared to other heavy metals. Lead can cross the placenta and blood-brain barrier, and it can be excreted through breast milk. During lactation, maternal bone resorption increases to meet heightened calcium demands, potentially leading to the transfer of lead from maternal bone stores to the blood and breast milk [32]. Lead in breast milk can account for 12% of an infant’s BLL, while the maternal BLL can contribute 30% [33]. Exposure to lead in children can negatively impact brain development, leading to a decrease in intelligence quotient, brain damage, and impaired physical growth [34].
- The CDC advises that BLLs for breastfeeding mothers should be under 5 μg/dL [15]. However, our study suggests that even lower levels can decrease serum prolactin concentrations. This is consistent with the 2016 findings of Dobrakowski et al. [35], who found that participants with chronic lead exposure had serum prolactin levels 41% lower than those of the control groups. While the exact mechanism by which BLL may affect serum prolactin levels is not yet fully understood, several animal studies have suggested that lead exposure can increase the number of dopamine transporters and D1 and D2 receptors [36]. The increase in dopamine transporters and receptors, particularly D2, inhibits the release of prolactin from lactotroph cells in the hypothalamus, resulting in lower blood prolactin levels. In contrast, the absence of the receptor has been linked to the development of hyperprolactinemia [37].
- In this study, several factors were found to be unrelated to serum prolactin levels, including maternal age, parity, and intake of vitamins and minerals, as well as the age and sex of the infant. Most respondents were healthy reproductive-age females, between 20 years and 30 years old who were exclusively breastfeeding. Roelfsema et al. [38] found that 24-hour prolactin concentration was associated with body mass index and sex, but not age. Similarly, parity did not significantly correlate with serum prolactin level. This finding contradicts that of Hill et al. [39], who reported that female with a history of multiple births have more prolactin receptors in their mammary glands than those with no prior births. The age and sex of the infant also did not influence prolactin levels. This contrasts with previous research, which indicated that serum prolactin levels were initially high at the onset of lactation and gradually decreased, yet remained comparatively high in breastfeeding women [40].
- Regarding the strengths of this study, the samples were gathered a day after the dietary assessment of the mother was finalized, and dietary measurements were conducted 3 times with a large number of samples. The use of ICP-MS for measuring BLL has been demonstrated to be particularly sensitive, at a level of parts per trillion. However, the cross-sectional analytic design precluded the possibility of drawing causal conclusions.
- The main finding of this study was that exposure to lead can decrease serum prolactin levels among lactating mothers. Upon examining the correlation between various maternal and infant factors, we determined that the frequency of breastfeeding has a greater effect on prolactin levels than the consumption of protein or calcium. Consequently, it is crucial for policymakers to devise specific interventions and management strategies for lactating mothers who have been exposed to lead.
DISCUSSION
-
CONFLICT OF INTEREST
The authors have no conflicts of interest associated with the material presented in this paper.
-
FUNDING
This project was provided by Universitas Brawijaya under grant No. 3127/UN10.F08/PN.2021.
-
AUTHOR CONTRIBUTIONS
Conceptualization: Wati LR, Sargowo D. Data curation: Wati LR, Nurseta T, Rahardjo B. Formal analysis: Sargowo D, Zuhriyah L, Wati LR. Funding acquisition: Nurseta T. Methodology: Zuhriyah L, Sargowo D. Visualization: Wati LR. Writing – original draft: Wati LR, Nurseta T, Sargowo D. Writing – review & editing: Sargowo D, Nurseta T, Zuhriyah L, Rahardjo B.
Notes
ACKNOWLEDGEMENTS
| Characteristics | Categories | Frequency (%) |
|---|---|---|
| Maternal | ||
| Age (y) | <20 | 2 (1.8) |
| 20–30 | 74 (67.3) | |
| 31–40 | 32 (29.1) | |
| >40 | 2 (1.8) | |
| Parity | Primipara | 31 (28.2) |
| Multipara | 72 (65.4) | |
| Grande multipara | 7 (6.4) | |
| Breastfeeding frequency (times/day) | 8–10 | 54 (49.1) |
| 11–17 | 48 (43.6) | |
| >17 | 8 (7.3) | |
| Education | Lower school | 78 (70.9) |
| High school or college | 32 (29.1) | |
| Length of residence in the research area (y) | <5 | 19 (17.3) |
| 5–10 | 28 (25.4) | |
| >10 | 63 (57.3) | |
| Monthly family income (rupiah)1 | Above minimum wage | 37 (33.6) |
| Below minimum wage | 73 (66.4) | |
|
|
||
| Infant | ||
| Sex | Male | 61 (55.4) |
| Female | 49 (44.5) | |
| Age (mo) | 1–3 | 68 (61.8) |
| >3–6 | 42 (38.2) | |
| Current weight (BMI) for age | Overweight | 6 (5.4) |
| Normal weight | 72 (65.5) | |
| Underweight | 32 (29.1) | |
| Variable | Maternal BLL (μg/dL) | p-value | Serum prolactin (ng/mL) | p-value |
|---|---|---|---|---|
| Total | 3.64±1.38 | 129.19±88.96 | ||
|
|
||||
| Maternal characteristics | ||||
| Age (y) | 0.995 | 0.073 | ||
| <20 | 3.75±1.48 | 84.80±67.17 | ||
| 20–30 | 3.62±1.42 | 133.01±85.67 | ||
| 31–40 | 3.70±1.34 | 112.07±88.76 | ||
| >40 | 3.50±1.13 | 305.90±35.21 | ||
| Parity | 0.442 | 0.561 | ||
| Primipara | 3.48±1.40 | 133.18±84.19 | ||
| Multipara | 3.77±1.37 | 128.12±88.88 | ||
| Grande multipara | 3.31±1.40 | 118.85±93.65 | ||
| Breastfeeding frequency (times/day) | 0.212 | <0.0013 | ||
| 8–10 | 3.51±1.39 | 71.35±42.88 | ||
| 11–17 | 3.83±1.37 | 174.55±80.61 | ||
| >17 | 3.00±1.33 | 320.20±7.42 | ||
| Education | 0.388 | 0.818 | ||
| Lower school | 3.69±1.35 | 128.86±91.42 | ||
| High school or college | 3.53±1.45 | 129.96±84.04 | ||
| Length of residence in the research area (y) | 0.758 | 0.888 | ||
| <5 | 3.44±1.17 | 126.87±79.29 | ||
| 5–10 | 3.80±1.72 | 124.94±93.77 | ||
| >10 | 3.68±1.34 | 131.63±92.54 | ||
| Monthly family income (rupiah)2 | 0.165 | 0.816 | ||
| Above minimum wage | 3.51±1.34 | 131.32±91.62 | ||
| Below minimum wage | 3.90±1.43 | 125.14±84.73 | ||
|
|
||||
| Maternal diet | ||||
| Protein (g) | 0.0193 | <0.0013 | ||
| <70 | 3.89±1.48 | 82.74±57.35 | ||
| 70–80 | 3.72±1.56 | 127.78±78.63 | ||
| >80 | 3.45±1.24 | 162.53±96.18 | ||
| Calories (kcal) | 0.348 | 0.103 | ||
| <1500 | 3.37±1.23 | 98.44±64.30 | ||
| ≥1500 | 3.71±1.41 | 136.87±92.83 | ||
| Vitamin B6 (mg/day) | 0.110 | 0.772 | ||
| <2 | 3.95±1.26 | 142.56±102.27 | ||
| ≥2 | 2.47±1.18 | 125.65±84.78 | ||
| Vitamin D (μg/day) | 0.210 | 0.895 | ||
| <5 | 3.82±1.30 | 127.44±87.05 | ||
| ≥5 | 3.31±1.47 | 132.48±93.56 | ||
| Vitamin E (mg/day) | 0.309 | 0.618 | ||
| <15 | 3.36±1.37 | 129.72±89.19 | ||
| ≥15 | 3.45±1.26 | 139.53±84.21 | ||
| Zinc (mg/day) | 0.070 | 0.189 | ||
| <16 | 3.71±1.35 | 132.26±89.74 | ||
| ≥16 | 2.92±1.54 | 96.61±75.46 | ||
| Folic acid (μg/day) | 0.115 | 0.224 | ||
| <500 | 3.70±1.36 | 131.25±89.78 | ||
| ≥500 | 3.52±1.29 | 140.76±94.27 | ||
| Calcium (mg/day) | <0.0014 | 0.0214 | ||
| <1000 | 3.85±1.29 | 126.75±89.12 | ||
| ≥1000 | 2.41±1.13 | 153.28±87.45 | ||
| Magnesium (mg/day) | 0.302 | 0.841 | ||
| <310 | 3.55±1.44 | 132.01±90.87 | ||
| ≥310 | 3.73±1.33 | 126.55±87.87 | ||
| Iron (mg/day) | 0.537 | 0.273 | ||
| <15 | 3.56±1.38 | 136.77±89.67 | ||
| ≥15 | 3.38±1.26 | 116.55±87.87 | ||
|
|
||||
| Infant characteristics | ||||
| Sex | 0.0094 | 0.153 | ||
| Male | 3.30 ±1.15 | 138.44±90.39 | ||
| Female | 4.06 ±1.53 | 117.65±86.67 | ||
| Age (mo) | 0.063 | 0.0063 | ||
| 1 | 2.71 ±1.23 | 176.90±104.24 | ||
| 2 | 3.83 ±1.45 | 150.26±86.27 | ||
| 3 | 3.64 ±1.21 | 141.45±94.19 | ||
| 4 | 3.53 ±1.01 | 80.73±38.22 | ||
| 5 | 3.97 ±1.26 | 94.22±78.28 | ||
| 6 | 4.62 ±1.97 | 79.61±49.20 | ||
| Current weight (BMI) for age | 0.417 | 0.094 | ||
| Overweight | 3.78±1.36 | 133.18±84.19 | ||
| Normal | 3.34±1.34 | 128.12±88.88 | ||
| Underweight | 3.72±1.42 | 118.85±93.65 | ||
Values are presented as mean±standard deviation.
BLL, blood lead level; BMI, body mass index.
1 Post-hoc analysis of relationship between protein intake and BLL using Dunn test: (<70 vs. 70–80, p=0.531; <70 vs. >80, p<0.001; 70–80 vs. >80, p=0.960); Post-hoc analysis of relationships of breastfeeding frequency, protein intake, and infant age with prolactin level using Dunn test: breastfeeding (8–10 vs. 11–17, p<0.001; 9–10 vs. >17, p<0.001; 11–17 vs. >17, p=0.187), protein intake (<70 vs. 70–80, p=0.131; <70 vs. >80, p<0.001; 70–80 vs. >80, p=0.783), and infant age (1 vs. 4, p=0.007; 1 vs. 5, p=0.007; 1 vs. 6, p=0.013; 2 vs. 4, p=0.017; 2 vs. 5, p=0.018; 2 vs. 6, p=0.031).
2 Minimum wage=rupiah 4.375.479.
3 Kruskal-Wallis test.
4 Mann-Whitney test.
| Models | β | Coefficient correlation | p-value | 95% CI | R2 | |
|---|---|---|---|---|---|---|
| LL | UL | |||||
| Breastfeeding frequency | 6.023 | 0.677 | <0.001 | 5.046 | 7.001 | 0.7751 |
| Protein intake | 1.866 | 0.218 | <0.001 | 2.784 | 0.947 | |
| Vitamin B6 | −2.226 | −0.169 | 0.003 | −3.693 | −0.759 | |
| Calcium | 0.124 | 0.139 | 0.011 | 0.029 | 0.219 | |
| BLL | −2.756 | −0.323 | <0.001 | −3.589 | −1.923 | |
- 1. World Health Organization. Infant and young child feeding; 2021 [cited 2023 Jul 5]. Available from: https://www.who.int/news-room/fact-sheets/detail/infant-and-young-child-feeding
- 2. Berens PD, Villanueva M, Nader S, Swaim LS. Isolated prolactin deficiency: a possible culprit in lactation failure. AACE Clin Case Rep 2018;4(6):e509-e512Article
- 3. Saleem M, Martin H, Coates P. Prolactin biology and laboratory measurement: an update on physiology and current analytical issues. Clin Biochem Rev 2018;39(1):3-16PubMedPMC
- 4. Al-Chalabi M, Bass AN, Alsalman I. Physiology, prolactin; 2022 [cited 2023 Jul 5]. Available from: https://www.ncbi.nlm.nih.gov/books/NBK507829/
- 5. Levine S, Muneyyirci-Delale O. Stress-induced hyperprolactinemia: pathophysiology and clinical approach. Obstet Gynecol Int 2018;2018: 9253083ArticlePubMedPMCPDF
- 6. Pitilin ED, Gasparin VA, Bagatini MD, Lentsck MH. Determinants of the prolactin level in imediate postpartum women. Cogit Enferm 2020;25: e71511
- 7. Phillipps HR, Yip SH, Grattan DR. Patterns of prolactin secretion. Mol Cell Endocrinol 2020;502: 110679ArticlePubMed
- 8. Tikk K, Sookthai D, Johnson T, Dossus L, Clavel-Chapelon F, Tjønneland A, et al. Prolactin determinants in healthy women: a large cross-sectional study within the EPIC cohort. Cancer Epidemiol Biomarkers Prev 2014;23(11):2532-2542ArticlePubMedPDF
- 9. Santoso M, Lestiani DD, Mukhtar R, Hamanongan E. Early warning of air quality status through characterization of PM2.5 lead concentration at several cities in Indonesia. Ecolab 2015;9(1):01-46. (Indonesian)
- 10. World Health Organization. Air pollution is responsible for 6, 7 million premature deaths every year; 2021 [cited 2023 Jul 25]. Available from: https://www.who.int/teams/environment-climate-change-and-health/air-quality-and-health/health-impacts/types-of-pollutants
- 11. Hutomo LP, Wulandari SY, Marwoto J. Distribution study of lead (Pb) and copper (Cu) concentrations in sediments at Kenjeran Beach, Surabaya. J Oseanogr 2016;5(2):277-285. (Indonesian)
- 12. Efanny M, Andarwulan N, Yuliana ND. Dietary exposure assessment and risk characterization of lead based on lead contaminant research (online) in Indonesia and Indonesian Individual Food Consumption Survey (IFCS). IOP Conf Ser Earth Environ Sci 2019;278: 012021ArticlePDF
- 13. Soegianto A, Putranto TW, Lutfi W, Almirani FN, Hidayat AR, Muhammad A, et al. Concentrations of metals in tissues of cockle Anadara granosa (Linnaeus, 1758) from East Java Coast, Indonesia, and potential risks to human health. Int J Food Sci 2020;2020: 5345162ArticlePubMedPMCPDF
- 14. Javorac D, Baralić K, Marić Đ, Mandić-Rajčević S, Đukić-Ćosić D, Bulat Z, et al. Exploring the endocrine disrupting potential of lead through benchmark modelling - study in humans. Environ Pollut 2023;316(Pt 1):120428ArticlePubMed
- 15. Centers for Disease Control and Prevention. Guidelines for the identification and management of lead exposure in pregnant and lactating women; 2010 [cited 2023 Jul 5]. Available from: https://www.cdc.gov/nceh/lead/publications/leadandpregnancy2010.pdf
- 16. Meeker JD, Rossano MG, Protas B, Diamond MP, Puscheck E, Daly D, et al. Multiple metals predict prolactin and thyrotropin (TSH) levels in men. Environ Res 2009;109(7):869-873ArticlePubMedPMC
- 17. Takser L, Mergler D, Lafond J. Very low level environmental exposure to lead and prolactin levels during pregnancy. Neurotoxicol Teratol 2005;27(3):505-508ArticlePubMed
- 18. Lemeshow S, Hosmer DW Jr, Klar J, Lwanga SK. Adequacy of sample size in health studies; 1990 [cited 2023 Jul 5]. Available from: https://www.academia.edu/39511442/Adequacy_of_Sample_Size_in_Health_Studies
- 19. Health Ministry of Republic Indonesia. Table of Indonesian food composition. Jakarta: Health Ministry of Republic Indonesia; 2018. p. 1-127 (Indonesian)
- 20. Mazarakioti EC, Zotos A, Thomatou AA, Kontogeorgos A, Patakas A, Ladavos A. Inductively coupled plasma-mass spectrometry (ICP-MS), a useful tool in authenticity of agricultural products’ and foods’ origin. Foods 2022;11(22):3705ArticlePubMedPMC
- 21. Pal S. Evaluation of the correlation coefficient of polyethylene glycol treated and direct prolactin results and comparability with different assay system results. EJIFCC 2017;28: 315-327PubMedPMC
- 22. Huang SK, Chih MH. Increased breastfeeding frequency enhances milk production and infant weight gain: correlation with the basal maternal prolactin level. Breastfeed Med 2020;15(10):639-645ArticlePubMed
- 23. Cregan MD, Mitoulas LR, Hartmann PE. Milk prolactin, feed volume and duration between feeds in women breastfeeding their full-term infants over a 24 h period. Exp Physiol 2002;87(2):207-214ArticlePubMedPDF
- 24. Kominiarek MA, Rajan P. Nutrition recommendations in pregnancy and lactation. Med Clin North Am 2016;100(6):1199-1215ArticlePubMedPMC
- 25. Velázquez-Villegas LA, López-Barradas AM, Torres N, Hernández-Pando R, León-Contreras JC, Granados O, et al. Prolactin and the dietary protein/carbohydrate ratio regulate the expression of SNAT2 amino acid transporter in the mammary gland during lactation. Biochim Biophys Acta 2015;1848(5):1157-1164ArticlePubMed
- 26. Syofyan SS, Wahyuni AS, Rusmil K, Lelo A. The effects of calcium supplementation on blood lead levels and short-term memory of chronically exposed children: a clinical trial study. Open Access Maced J Med Sci 2020;8(B):1144-1151ArticlePDF
- 27. Zhuo C, Xu Y, Wang H, Fang T, Chen J, Zhou C, et al. Safety and efficacy of high-dose vitamin B6 as an adjunctive treatment for antipsychotic-induced hyperprolactinemia in male patients with treatment-resistant schizophrenia. Front Psychiatry 2021;12: 681418ArticlePubMedPMC
- 28. Kok P, Roelfsema F, Langendonk JG, de Wit CC, Frölich M, Burggraaf J, et al. Increased circadian prolactin release is blunted after body weight loss in obese premenopausal women. Am J Physiol Endocrinol Metab 2006;290(2):E218-E224ArticlePubMed
- 29. Gillis BS, Arbieva Z, Gavin IM. Analysis of lead toxicity in human cells. BMC Genomics 2012;13: 344ArticlePubMedPMCPDF
- 30. RÍsovÁ V. The pathway of lead through the mother’s body to the child. Interdiscip Toxicol 2019;12(1):1-6ArticlePubMedPMC
- 31. Rebelo FM, Caldas ED. Arsenic, lead, mercury and cadmium: toxicity, levels in breast milk and the risks for breastfed infants. Environ Res 2016;151: 671-688ArticlePubMed
- 32. Samiee F, Vahidinia A, Taravati Javad M, Leili M. Exposure to heavy metals released to the environment through breastfeeding: a probabilistic risk estimation. Sci Total Environ 2019;650(Pt 2):3075-3083ArticlePubMed
- 33. Ettinger AS, Téllez-Rojo MM, Amarasiriwardena C, Bellinger D, Peterson K, Schwartz J, et al. Effect of breast milk lead on infant blood lead levels at 1 month of age. Environ Health Perspect 2004;112(14):1381-1385ArticlePubMedPMC
- 34. Irawati Y, Kusnoputranto H, Achmadi UF, Safrudin A, Sitorus A, Risandi R, et al. Blood lead levels and lead toxicity in children aged 1–5 years of Cinangka Village, Bogor Regency. PLoS One 2022;17(2):e0264209ArticlePubMedPMC
- 35. Dobrakowski M, Kasperczyk A, Czuba ZP, Machoń-Grecka A, Szlacheta Z, Kasperczyk S. The influence of chronic and subacute exposure to lead on the levels of prolactin, leptin, osteopontin, and follistatin in humans. Hum Exp Toxicol 2017;36(6):587-593ArticlePubMedPDF
- 36. Khotimah H, Wari FE, Noviasari D, Octaviana A, Supriadi RF, Norisa N, et al. Centella asiatica alleviates neurotoxicity and development of lead-exposed zebrafish larvae. Aquac Aquar Conserv Legis 2020;13(4):1886-1898
- 37. Al-Kuraishy HM, Al-Gareeb AI, Butnariu M, Batiha GE. The crucial role of prolactin-lactogenic hormone in Covid-19. Mol Cell Biochem 2022;477(5):1381-1392ArticlePubMedPMCPDF
- 38. Roelfsema F, Pijl H, Keenan DM, Veldhuis JD. Prolactin secretion in healthy adults is determined by gender, age and body mass index. PLoS One 2012;7(2):e31305ArticlePubMedPMC
- 39. Hill PD, Chatterton RT Jr, Aldag JC. Serum prolactin in breastfeeding: state of the science. Biol Res Nurs 1999;1(1):65-75ArticlePubMedPDF
- 40. Boss M, Gardner H, Hartmann P. Normal human lactation: closing the gap. F1000Res 2018;7: F1000 Faculty Rev-801ArticlePDF
REFERENCES
Figure & Data
References
Citations

 KSPM
KSPM

 PubReader
PubReader ePub Link
ePub Link Cite
Cite


