Articles
- Page Path
- HOME > J Prev Med Public Health > Volume 49(4); 2016 > Article
-
Review
Short-term Effect of Fine Particulate Matter on Children’s Hospital Admissions and Emergency Department Visits for Asthma: A Systematic Review and Meta-analysis -
Hyungryul Lim1
 , Ho-Jang Kwon1
, Ho-Jang Kwon1 , Ji-Ae Lim1
, Ji-Ae Lim1 , Jong Hyuk Choi1
, Jong Hyuk Choi1 , Mina Ha1
, Mina Ha1 , Seung-Sik Hwang2
, Seung-Sik Hwang2 , Won-Jun Choi3
, Won-Jun Choi3 -
Journal of Preventive Medicine and Public Health 2016;49(4):205-219.
DOI: https://doi.org/10.3961/jpmph.16.037
Published online: July 14, 2016
1Department of Preventive Medicine, Dankook University College of Medicine, Cheonan, Korea
2Department of Social and Preventive Medicine, Inha University School of Medicine, Incheon, Korea
3Department of Occupational and Environmental Medicine, Gachon University Gil Medical Center, Incheon, Korea
- Corresponding author: Ho-Jang Kwon, MD, PhD 119 Dandae-ro, Dongnam-gu, Cheonan 31116, Korea Tel: +82-41-550-3879, Fax: +82-41-556-6461 E-mail: hojang@dankook.ac.kr
Copyright © 2016 The Korean Society for Preventive Medicine
This is an Open Access article distributed under the terms of the Creative Commons Attribution Non-Commercial License (http://creativecommons.org/licenses/by-nc/4.0/) which permits unrestricted non-commercial use, distribution, and reproduction in any medium, provided the original work is properly cited.
ABSTRACT
-
Objectives:
- No children-specified review and meta-analysis paper about the short-term effect of fine particulate matter (PM2.5) on hospital admissions and emergency department visits for asthma has been published. We calculated more precise pooled effect estimates on this topic and evaluated the variation in effect size according to the differences in study characteristics not considered in previous studies.
-
Methods:
- Two authors each independently searched PubMed and EMBASE for relevant studies in March, 2016. We conducted random effect meta-analyses and mixed-effect meta-regression analyses using retrieved summary effect estimates and 95% confidence intervals (CIs) and some characteristics of selected studies. The Egger’s test and funnel plot were used to check publication bias. All analyses were done using R version 3.1.3.
-
Results:
- We ultimately retrieved 26 time-series and case-crossover design studies about the short-term effect of PM2.5 on children’s hospital admissions and emergency department visits for asthma. In the primary meta-analysis, children’s hospital admissions and emergency department visits for asthma were positively associated with a short-term 10 μg/m3 increase in PM2.5 (relative risk, 1.048; 95% CI, 1.028 to 1.067; I2=95.7%). We also found different effect coefficients by region; the value in Asia was estimated to be lower than in North America or Europe.
-
Conclusions:
- We strengthened the evidence on the short-term effect of PM2.5 on children’s hospital admissions and emergency department visits for asthma. Further studies from other regions outside North America and Europe regions are needed for more generalizable evidence.
- The adverse health effects of air pollution on respiratory and cardiovascular diseases are well known to the public. Regulation and monitoring of air pollution are performed at both the national and international levels. Particulate matter (PM) is one type of air pollutant. It is not a specific chemical entity, unlike other commonly known pollutants such as ozone, sulphur dioxide, and nitrogen dioxide. It is a physical category of dust with different components mixed together [1]. The particle size determines the different categorizations: PM10 (less than 10 μm aerodynamic diameter) and PM2.5 (less than 2.5 μm aerodynamic diameter). PM2.5 is also known as fine PM.
- PM2.5 has been reported to play a major role in increasing the chance of mortality due to cardiovascular diseases because it can penetrate the capillary vessel of the lungs and reach the alveoli [2,3]. Extensive research has been conducted on the association between PM2.5 and respiratory diseases including asthma. Asthma is a syndrome in which reversible respiratory obstruction occurs and is characterized by hypersensitiveness to allergens. When stimulated, a person experiences wheezing and dyspnea. In most cases, asthma is caused by a genetic predisposition and is triggered by environmental allergens.
- The prevalence rate of asthma is high in children. In the case of South Korea (hererafter Korea), the prevalence rate of asthma in children steadily increased due to urbanization and westernization. In 2010, a national study based on the International Study of Asthma and Allergies in Childhood questionnaire found that 10.1% of elementary school students and 8.5% of middle school students had experienced symptoms of asthma in the past 12 months [4]. These numbers should not be ignored.
- Recently published systematic reviews and meta-analyses reported the pooled relative risk (RR) of the number of hospital admissions and emergency department (ED) visits due to asthma as 1.023 (95% confidence interval [CI], 1.015 to 1.031, per 10 μg/m3 increase) when examining the effects of PM2.5 on the total population, and 1.025 (95% CI, 1.013 to 1.037, per 10 μg/m3 increase) when the subject was confined to children only [5]. Another review that examined the effects of PM2.5 on ED visits due to asthma reported a pooled RR of 1.036 (95% CI, 1.018 to 1.053, per 10 μg/m3 increase) [6]. However, existing studies contain several limitations. These studies were not focused on childhood asthma and only presented pooled effect estimates in children as subgroup analysis. Moreover, most of the relevant studies were conducted in North America and Europe [7-28], and although studies conducted in other regions exist [29-32], they did not consider the varying effects of PM2.5 according to different regions. The design of the study, the background PM2.5 mean concentration and variation of the region where the study was conducted, and the time of study may change the effects as well, but these factors were not adequately considered in existing studies.
- In addition to the two reviews mentioned above, seven new relevant papers have recently been published [22-28]. Of these, the time-series studies assessed the exposure to air pollution by using the exposure value of the population-weighted average in between the measuring points of air pollution [22,24,27], and the case-crossover design studies used the method of matching individual addresses with the PM2.5 measures [25,26], which yielded more accurate results. Therefore, by including these recent developments, we tried to calculate more accurate pooled effect estimates of the effects of PM2.5 on childhood asthma and assess the variations of effects induced by differences in some factors such as region or date of research, which have been not adequately examined yet.
INTRODUCTION
- Selection Criteria
- We first determined some criteria for selecting relevant studies. They are as follows:
1) The subject of study was limited to children and adolescents under the age of 20.
2) Study results were limited to computerized records of hospital admissions and ED visits. Outpatient visits were excluded. Hospital admissions confirmed through interviews were not eligible. Subjective symptoms, decrease in pulmonary function, and use of emergency inhalers were not considered endpoints.
3) Effect estimates had to be presented as an odds ratio (OR) or RR.
- Search Terms and Study Selection
- When deciding on search terms, we minimized keywords in order to increase the sensitivity of our searches. Some of the search terms we used were child*, pediatric*, fine particulate matter*, fine particle*, PM2.5, asthma*, hospitalization, hospitalisation, admission*, ed, er, and emergency. We searched studies to include in our meta-analysis using PubMed and EMBASE in March of 2016. Moreover, we selected the final eligible studies after having two authors each independently select references according to the criteria above and the same search terms and then comparing the two lists.
- Statistical Methods
- The effect size was expressed as RR. We considered the OR as a proxy to the RR. In order to have all the effect estimates chosen from the selected studies to reflect the same 10 μg/m3 increment of PM2.5 concentration, we implemented meta-analyses after recalculating the β coefficient and 95% CI presented in each study. Because the purpose of this study is to combine and identify the effects from regions all over the world, generalization of heterogeneous parts of the research group was its goal. Therefore, the random effects model using the DerSimonian and Laird [33] estimation method was mainly considered, rather than the fixed effects model [33,34]. When estimating the pooled effect, the model takes into account both the between-study variation and the within-study variation and provides a greater confidence level than the fixed effects model. The I-squared value (%) was calculated in order to identify heterogeneity.
- In the primary meta-analysis of this study, an effect estimate that could represent the selected studies was used. We used the same lag value that was presented in the original paper [35], but if a study presented multiple estimates from different lags, we selected the one with the largest effect size. This is because, generally, these works report the greatest effect size [36]. If a study did not have one effect estimate that could represent the research, we selected two or more values that were obtained from subjects that were mutually exclusive (that is, if a study did not present an effect estimate in whole participants but presented two or more separate values from stratified groups, we included those in the meta-analysis). In order to identify publication bias, we conducted the Egger’s test and identified the degree of asymmetry through a funnel plot [37].
- Moreover, we conducted category-specific meta-analyses in order to determine what factors influenced the effect of PM2.5, if those influences were robust, and what factors contributed to the heterogeneity of effect estimates. We conducted the analyses by sorting the effect estimates into categories of age, results (records of hospital admissions or ED visits), season, design of the study, region, and the lag of exposure. We also conducted a separate analysis according to whether or not different pollutants were adjusted in the statistical model.
- We hypothesized that the components of PM2.5 would change according to the time of the study and that the size of the effect could change according to the components. In addition, we thought that the variation and the mean concentration of PM2.5 in the region where the study was conducted might change the size of the effect. Therefore, through mixed-effects meta-regression, we derived an effect estimate of the time of the study, and the mean and standard deviation of the concentration in the study region on RR for childhood asthma.
- All statistical analyses performed using R version 3.1.3 (Comprehensive R Archive Network: http://cran.r-project.org) and we carried out a series of statistical analyses described above through the meta package. All statistical analyses set a 5% significance level for the two-tailed test.
METHODS
- Selection of Relevant Studies and Extracting Effect Estimates and Their Confidence Intervals
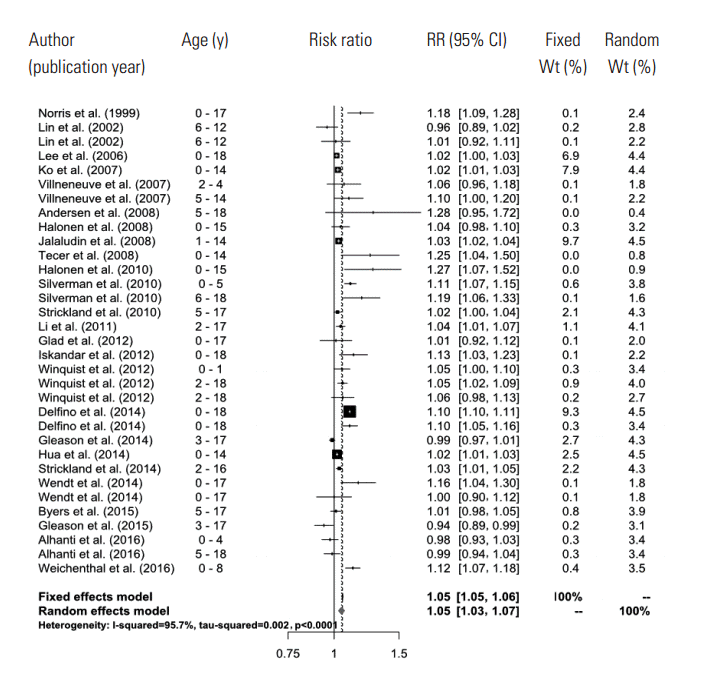
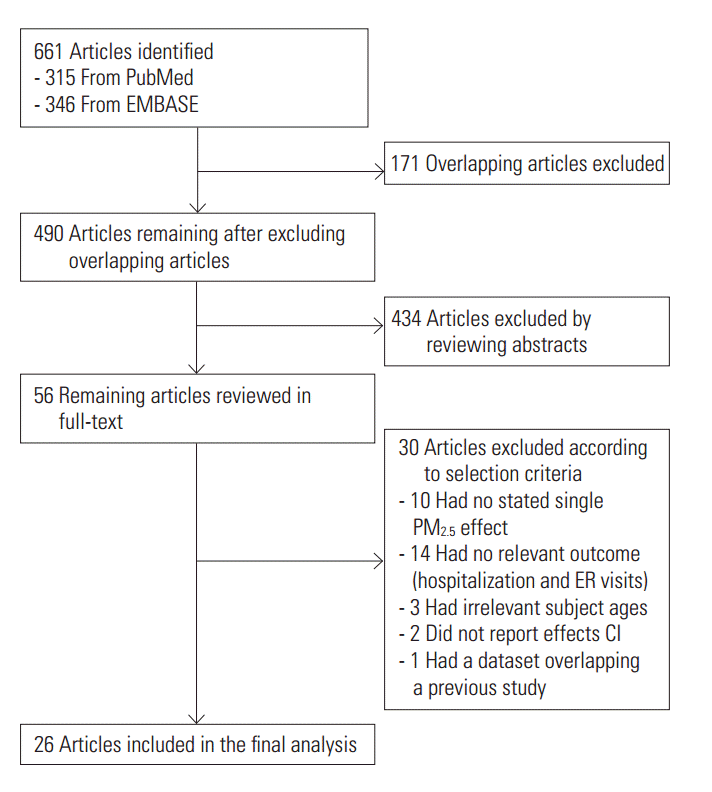
- A total of 661 references were searched using the search terms mentioned above, and of those, we first selected 56 to examine in whole by excluding overlapping studies (n=171) and reading the titles and abstracts (n=490). Then we ultimately selected 26 studies according to the selection criteria and extracted effect estimates (Figure 1). The 26 studies were published between 1999 and 2016, and we summarized each of the research outlines and the main research results in Table 1. Most of the research was conducted in North America and Europe and both time-series and case-crossover designs were almost equally represented.
- After extracting all effect estimates and CIs from the main body of each research paper and its supplementary materials, we broke it down to a total of 244 effect estimates. Of those, we selected 33 representative effect estimates from each study to use in our primary meta-analysis.
- Primary Meta-analysis
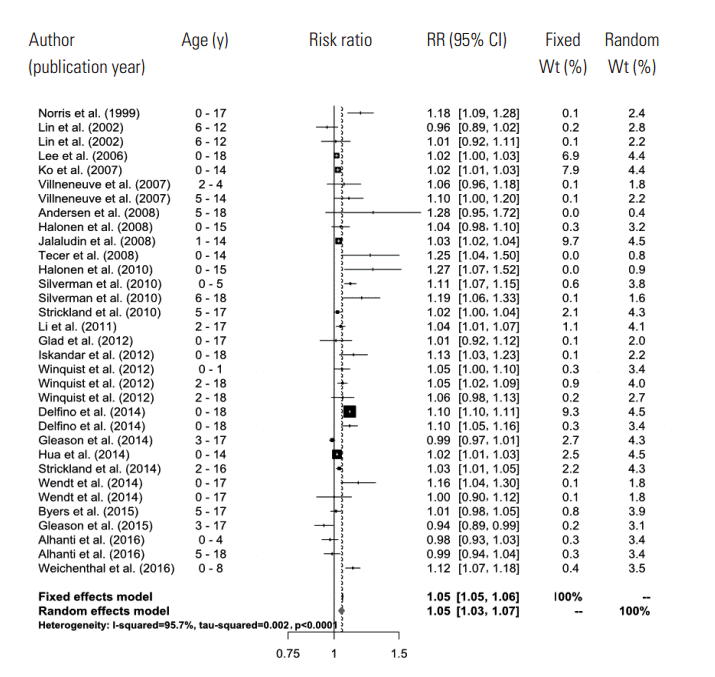
- In the random effects model, we were able to find that when the concentration of PM2.5 increased by 10 μg/m3, the risk of a child’s hospital admission or ED visit increased by 4.8% (RR, 1.048; 95% CI, 1.028 to 1.067). The I-squared value, which shows the heterogeneity of the included studies, was 95.6%, a high figure. We presented a forest plot for the included effect estimates and pooled estimates (Figure 2).
- Publication Bias
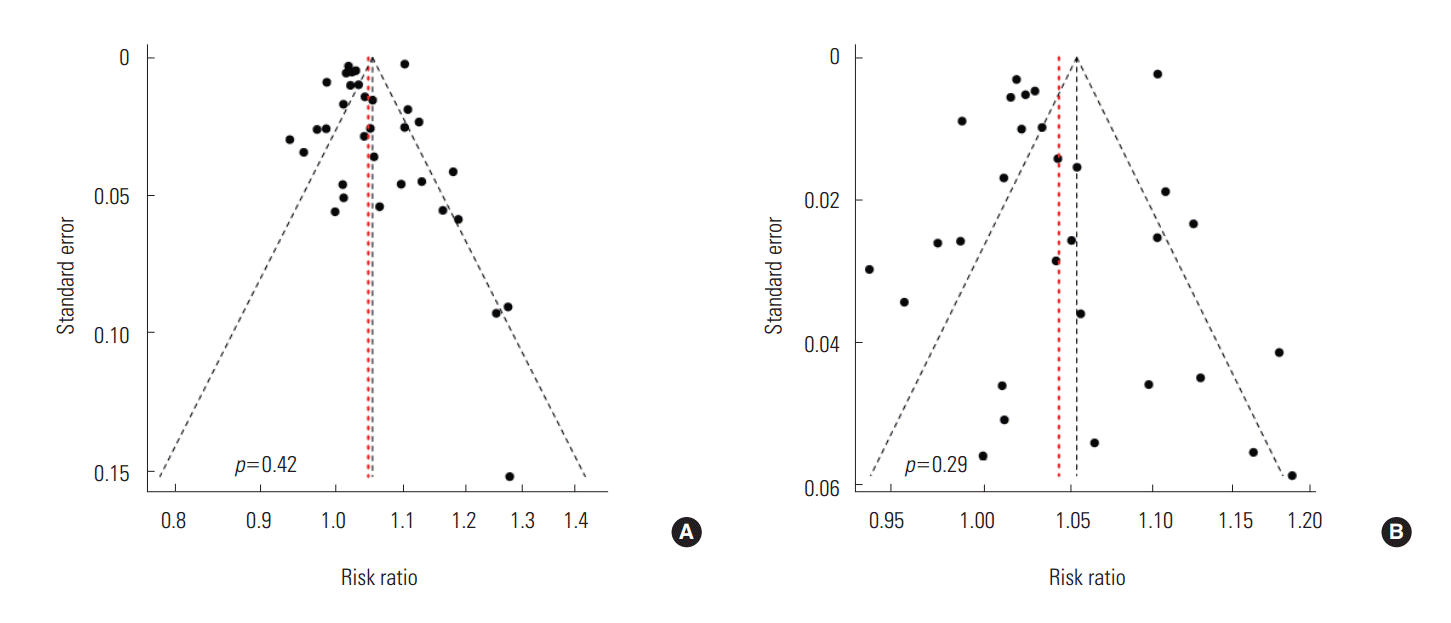
- To schematically examine the tendency toward publication bias, we found a relatively symmetrical shape in the funnel plot and confirmed that there was not much of a bias because there was not statistically significant (p=0.42) in the Egger ‘s test (Figure 3).
- Category-specific Meta-analyses
- We found that the effects are greater on children below the age of five than on children ages 5 to 19, in warmer seasons, and in North America and Europe than in Asia. The pooled effect estimates extracted through the multi-pollutant model was also statistically significant (RR, 1.040; 95% CI, 1.022 to 1.057). According to the lags, the effect changed greatly from 0.2% to 6.5%, and the effect was large for 3-day lag and 3-day average lag (Table 2).
- Meta-regression Analyses
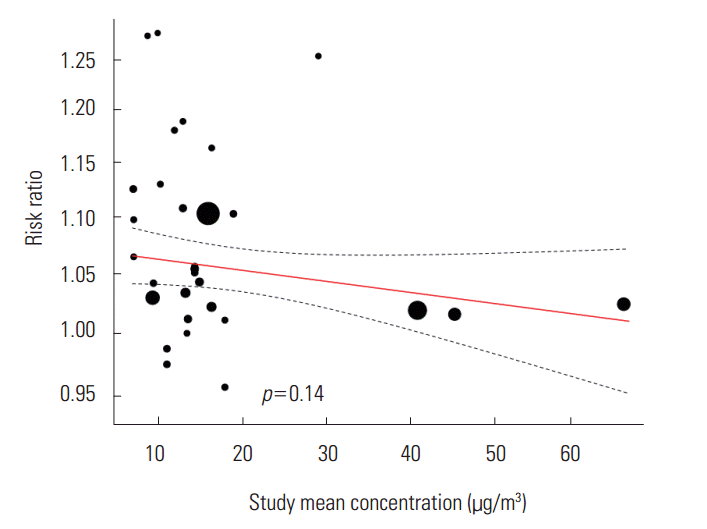
- We did not find a tendency toward change in the statistically significant RR according to the time of study and the standard deviation of the background concentration of the region of study. We found a negative tendency in the mean PM2.5 concentration by the region of study, but it was not statistically significant (β=-0.0008, p=0.14) (Figure 4).
RESULTS
- In the primary meta-analysis of the effect estimates obtained from the 26 studies, we found that in the short-term, when the concentration of PM2.5 increased 10 μg/m3, the risk of a child’s hospital admission or ED visit increased 4.8%, which is statistically significant. The effect of PM2.5 could be considered quite robust, since the effect was maintained to 4.0% even when we pooled the estimates extracted by the multi-pollutant model in this study. This number is greater than the 2.3% found among the total population presented in the aforementioned study of Zheng et al. [5]. These results show that children are more vulnerable to air pollution because their alveoli and airways are still growing, their immune systems are underdeveloped, and they spend more time outdoors, which increases ventilation [38].
- Based on known biological mechanisms, the generation of reactive oxygen species (ROS) is accelerated because of the transition metal included in PM2.5. Oxidative stress from ROS may be related to epithelial cell destruction and allergic inflammation, and this process is known to be related to exacerbation of asthma [39]. Meanwhile, previous studies reported that arginase may participate in a process that fine particles exacerbate childhood asthma [40]. In vivo studies report that the overexpression of arginase influences the hyperresponsiveness of airways [41] and that fine particles exacerbate the airway’s responsiveness in asthma in murine models [42]. Human epidemiological studies have shown that the variation of the ARG1 and ARG2 genes—which are related to the manifestation of arginase in childhood asthma patients—is statistically significant [40,43].
- In the preceding meta-analyses by Zheng et al. [5], they suggested 20 relevant studies on children’s asthma. We found a discrepancy between the selected studies of Zheng et al. [5] and ours even aside from seven papers published more recently. They cited several studies that we excluded in the process of extracting eligible studies. On the other hand, the six studies included in this study were not cited by the preceding study. We selected studies and extracted results carefully focusing on children. Therefore, we believe that the 26 references selected for this study comprise the best selection.
- We found that when the concentration of PM2.5 increased by 10 μg/m3, the risk of a child’s hospital admission or ED visit increased by 4.8%. This value is greater than the 2.5% increase in children found in the preceding meta-analysis by Zheng, et al. [5]. The following are some reasons to explain this difference. First, the newly added original studies included several studies in which the RR exceeded 1.10 when the measure of effect estimates was converted to 10 μg/m3 per increase [7,9,13,20,23,28]. Second, while the previous study pooled the effect estimates from the 0-day, 1-day, or 2-day average lags, we used the model with the greatest effect size out of the lags reported in the original studies.
- In this study, we found a difference in RR according to the season, and during the warmer seasons, the RR was 1.085 (95% CI, 1.051 to 1.119). The studies included in our meta-analysis showed quite consistent results [9,20,22,23,31]. We thought the reason for this was that during warmer seasons, children spend more time outdoors and therefore spend more time exposed to PM2.5. In addition, greater ventilation of buildings during these seasons makes it easier for air pollutants to penetrate inside the buildings. It was reported that the individual exposure concentration of PM2.5 that people living in well-ventilated environments showed high correlation to the concentration of the atmosphere [44]. The difference in components of PM2.5 according to the season may also be related, but because the extent of heterogeneity by region is too great, the evidence is not yet definitive [45-47].
- In terms of the design of the studies, the pooled RRs for the time-series and the case-crossover design studies were 1.028 and 1.051, respectively. For the case-crossover design, the OR was calculated using the conditional logistic regression model. Compared to the RR, the OR has a tendency to overestimate the actual risk. However, it may be thought as a closer representation of reality than the exposure assessment of the time-series because a recently published case-crossover study more accurately matched air pollutants using the addresses of individuals [20,21,25,26]. Residential information of patients entering hospitals or visiting the ED cannot be reflected in time-series. If we suppose that PM2.5 having an influence on exacerbating asthma as true, even in one study region, there is a possibility that the large effect in certain area with a high concentration could be diluted because of smaller effects in other area with a low concentration. We think that the actual effect is somewhere between the RRs of the time-series studies and the ORs of the case-crossover studies.
- When we examine the pooled RR of each lag, we can see that there is up to a 6% difference in value depending on the type of model. The effects of both the concentration three days before (3-day lag) and the average concentration over three days (3-day average lag) were considerable. This result is somewhat difficult to interpret. We need to consider the following factors when dealing with lags: that the ethnicities of the subject of study differ by regions and an accessibility to health services could change depending on the time of study. Through meta-regression analysis, we found a negative tendency among effect sizes depending on the mean concentration of PM2.5, but it was not statistically significant. Aside from the three studies in China which the mean concentrastion exceeded 30 μg/m3 (Figure 4), we did not find a negative tendency in the meta-regression analysis (β=-0.0004, p=0.90). Therefore, we could not draw conclusions in this study regarding such a limited tendency. A negative tendency means that the effect on asthma is smaller for regions where the mean concentrations of PM2.5 are higher. This means that the relationship between the mean concentration and the childhood asthma could be non-linear, or more specifically, supra-linear.
- When we examine the results according to region in the category-specific analyses, the pooled RR of the three studies conducted in Shanghai and Hong Kong was 1.019, which is a smaller value than those in North America (1.047) and Europe (1.075). This is similar to the results of the previous meta-analysis that examined the short-term effects of PM2.5 on total mortality and cardiorespiratory mortality, and found that the pooled estimate in China was lower than in the US, Europe, Japan, and Australia [48]. A hypothesis that the components of PM2.5 in China are different from those of developed countries was raised regarding this finding. In other words, in China, the contributions of coal combustion and desert dust—rather than exhaust from automobiles—were greater than in other regions.
- However, in a preceding meta-regression analysis including studies on PM10 and cardiorespiratory mortality conducted in China only, a statistically significant negative tendency was reported regarding the association between the mean concentrations of study regions and the effect sizes [49]. A study conducted across 27 US regions also reported that the effect of PM2.5 was greater in regions with lower background mean concentration, even though the result was not statistically significant [50]. In a cohort study on the effect of PM2.5 on cardiorespiratory mortality, the risks with the concentration level formed a supra-linear shape [2]. Therefore, for the regional effect variation in this study, the hypothesis that the effect was lowered in high concentrations seems more plausible, since only groups with resistance remain and detrimental effects on individuals vulnerable to PM2.5 occur in lower concentrations.
- There are many other genetic and environmental factors reported to cause childhood asthma besides PM2.5. Another hypothesis, following hygiene theory, states that allergic reactions decrease when children are exposed to micro-organisms because immune reactions are suppressed. Since westernization is still in progress in China, the effects of PM2.5 on asthma may be small [51,52]. There may be an objection to this statement since the three Chinese studies included in this study were conducted in Shanghai and Hong Kong, two very westernized large cities, but the infrastructure of the residences and the lifestyles of children growing up in such regions are different from those of North America and Europe.
- There are some limitations of this study. First, outpatient visits, use of inhalers, and other symptom outbreaks could all be considered health effects and consequences, but we confined the results to hospital admissions and ED visits which were mainly reported in previous studies. Therefore, the pooled effect estimate reported in our study might be underestimated. But in a study that uses surveys on symptoms and use of inhalers, the period between the exposure and outbreak could be imprecise. Moreover, results from a survey could be subjective. In cases of outpatient visits, we cannot exclude periodic follow-up cases. Second, we combined the RRs with the ORs because we deemed the OR to be proxy to the RR. Because of this, we may have calculated an overestimated value rather than the actual risk. However, in the case of Korea, hospitalization due to asthma among children between the ages of zero to 19 was 0.14% in 2014 [53]. The frequency of hospital admissions or ED visits due to asthma is rare so a possible bias will be negligible. Third, we could not control the innate heterogeneity of the selected studies. Components of PM2.5, ethnicities of the study population, and accessibility to health service as well as different age range, season, and adjusting variables or parameters in statistical models all probably affected the heterogeneity of the studies. However, we did not find a significant decrease of heterogeneity (Table 2). In order to obtain a more accurate pooled effect estimate, a meta-analysis should be conducted after an in-depth examination of the methods and quality of research.
- The strength of this study is that we newly included seven recent studies in our meta-analysis. In addition, with a focus on children, we examined variations in effect of different possible factors, and presented the direction for future studies. In particular, we raised the need for an epidemiological study on regions besides China with high concentrations of PM2.5.
DISCUSSION
- We found that in the short-term, when the concentration increased by 10 μg/m3, the risk of a child’s hospital admission or ED visit increased by 4.8%. If we consider the fact that air pollution affects a vast range of regions and many populations, this is not a negligible figure. A more fundamental solution is the reduction of the matter from emission sources, so we need to conduct studies on sources that emit PM2.5 and draft feasible environment-friendly policies for such emission sources.
CONCLUSION
ACKNOWLEDGEMENTS
-
CONFLICT OF INTEREST
The authors have no conflicts of interest associated with the material presented in this paper.
Notes


| Author (publication year) [Ref] | Study period | Location | Sample | Exposure assessment | Outcome | Study design | Statistical model | PM25 arithmetic mean concentration (μg/m3)(SD) | Major effect estimates (risk ratio) (95% CIs) |
|---|---|---|---|---|---|---|---|---|---|
| Norris et al. (1999) [7] | Sep 1, 1995-Dec 31, 1996 | Seattle, USA | <18y, 900 patients | 3 Fixed sites; a daily arithmetic mean was calculated and used | ED visits | TS | GAM with Poisson distribution | 12.0 (9.5) | Single-pollutant model |
| 1.15 (1.08, 1.23) for 1-d lag IQR increase | |||||||||
| Multi-pollutant model with SO2 and NO2 | |||||||||
| 1.17 (1.08, 1.26) for 1-d lag IQR increase | |||||||||
| Lin et al. (2002) [8] | Jan 1, 1981-Dec 31, 1993 | Toronto, Canada | 6-12 y, 7319 (boys: 4629, girls: 2690) patients | 1 Fixed site; the authors obtained data on every 6-d period from 1984 to 1990 and instructed a daily predicted value via modeling | HA | TS and CCD | GAM and conditional logistic regression | 18.0 (8.5) | Single-pollutant model |
| (a) Boys, | |||||||||
| 1.00 (0.97, 1.04) for the same day IQR increase in TS | |||||||||
| 1.01 (0.97, 1.06) for the same day IQR increase in CCD | |||||||||
| (b) Girls, | |||||||||
| 1.06 (0.99, 1.13) for 5-d average IQR increase in TS | |||||||||
| 1.04 (0.95, 1.15) for 5-d average IQR increase in CCD | |||||||||
| Multi-pollutant model with CO, SO2, NO2 and O3 | |||||||||
| (a) Boys, | |||||||||
| 0.96 (0.90, 1.02) for 5-d average IQR increase in TS | |||||||||
| 0.94 (0.85, 1.03) for 5-d average IQR increase in CCD | |||||||||
| (b) Girls, | |||||||||
| 1.01 (0.93, 1.10) for 5-d average IQR increase in TS | |||||||||
| 0.96 (0.85, 1.09) for 5-d average IQR increase in CCD | |||||||||
| Lee et al. (2006) [29] | Jan 1, 1997-Dec 31, 2002 | Hong Kong, China | ≤18 y, 26 663 patients | 13 Fixed sites (before 2000, 11 sites); a daily arithmetic mean was calculated and used | HA | TS | GAM with Poisson distribution | 45.3 (16.2) | Single-pollutant model |
| 1.066 (1.045, 1.087) for 4-d lag IQR increase | |||||||||
| Multi-pollutant model with CO, SO2, NO2 and O3 | |||||||||
| 1.032 (1.009, 1.056) for 1-d lag IQR increase | |||||||||
| Ko et al. (2007) [30] | Jan 1, 2000-Dec 31, 2005 | Hong Kong, China | ≤14 y, 23 596 patients | 3 Fixed sites; a daily arithmetic mean was calculated and used | HA | TS | GAM with Poisson distribution | 65.4 (21.1) | Single-pollutant model |
| 1.024 (1.013, 1.034) for 5-d average 10 μg/m3 increase | |||||||||
| Villneneuve et al. (2007) [9] | Jan 1, 1998-Mar 31, 2002 | Edmonton, Canada | 2-4 y, 7247 patients; | 3 Fixed sites; a daily arithmetic mean was calculated and used | ED visits | CCD | Conditional logistic regression | 7.01 in Apr to Sep; 7.31 in Oct to Mar | Single-pollutant model: |
| 5-14 y, 13 145 patients | (a) 2-4 y, | ||||||||
| 1.06 (0.97, 1.15) for 5-d average IQR increase | |||||||||
| - Oct to Mar: 0.95 (0.84, 1.07) | |||||||||
| - Apr to Sep: 1.16 (1.04, 1.28) | |||||||||
| (b) 5-14 y, | |||||||||
| 1.06 (1.00, 1.12) for 5-d average IQR increase | |||||||||
| - Oct to Mar: 0.99 (0.91, 1.09) | |||||||||
| - Apr to Sep: 1.10 (1.02, 1.17) | |||||||||
| Andersen et al. (2008) [10] | Oct 3, 2003-Dec 31, 2004 | Copenhagen, Denmark | 5-18 y, 559 patients in single pollutant model; 318 patients in two-pollutant model | 1 Fixed site; a daily arithmetic mean was calculated and used | HA | TS | GLM with Poisson regression | 10.0 (5.0) | Single-pollutant model |
| 1.15 (1.00, 1.32) for 6-d average IQR increase | |||||||||
| Two-pollutant model with total number concentration of particles | |||||||||
| 1.13 (0.98, 1.32) for 6-d average IQR increase | |||||||||
| Halonen et al. (2008) [11] | Jan 1, 1998-Dec 31, 2004 | Helsinki, Finland | <15y, 4807 patients | Fixed monitoring site, no specific information available | ED visits | TS | GLM with Poisson regression | 9.51 | Single-pollutant model |
| 1.026 (0.083, 1.054) for 4-d lag IQR increase | |||||||||
| Jalaludin et al. (2008) [31] | Jan 1, 1997-Dec 31, 2001 | Sydney, Australia | 1-14y, 317 724 patients | 14 Fixed sites; a daily arithmetic mean was calculated and used | ED visits | CCD | Conditional logistic regression | 9.4 (5.1) | Single-pollutant model |
| (a) 1-4 y, | |||||||||
| 1.014 (1.007, 1.021) for the same-day IQR increase | |||||||||
| - Warm months: 1.009 (1.002, 1.017) | |||||||||
| - Cool months: 1.010 (0.999, 1.024) | |||||||||
| (b) 5-9 y, | |||||||||
| 1.016 (1.005, 1.027) for the same-day IQR increase | |||||||||
| - Warm months: 1.013 (1.003, 1.024) | |||||||||
| - Cool months: 0.995 (0.976, 1.015) | |||||||||
| (c) 10-14 y, | |||||||||
| 1.012 (.0998, 1.027) for the same-day IQR increase | |||||||||
| - Warm months: 1.001 (0.987, 1.024) | |||||||||
| - Cool months: 1.017 (0.991, 1.044) | |||||||||
| Two-pollutant model with NO2 | |||||||||
| (a) 1-4 y, | |||||||||
| 1.008 (1.001, 1.015) for the same-day IQR increase | |||||||||
| (b) 5-9 y, | |||||||||
| 1.016 (1.006, 1.026) for the same-day IQR increase | |||||||||
| (c) 10-14 y, | |||||||||
| 1.011 (0.999, 1.024) for the same-day IQR increase | |||||||||
| Tecer et al. (2008) [12] | Dec 31, 2004-Oct 31, 2005 | ZiDnguldak, Turkey | <15y, 187 patients | 1 Fixed site; a daily arithmetic mean was calculated and used | HA | CCD | Conditional logistic regression | 29.1 (NA) | Single-pollutant model |
| 1.25 (1.05, 1.50) for 4-d lag 10 μg/m3 increase | |||||||||
| 1.37 (1.06, 1.76) for 4-d lag IQR increase | |||||||||
| Halonen et al. (2010) [13] | Jan 1, 1998-Dec 31, 2004 | Helsinki, Finland | Restricted to the warm season (May to Sep) | 2 Fixed sites; a daily arithmetic mean was calculated and used | ED visits | TS | GAM with Poisson distribution | 8.81 | Two-pollutant model with O3 |
| <15 y, 1972 patients | 1.148 (1.038, 1.270) for 5-d average IQR increase | ||||||||
| Silverman et al. (2010) [14] | Jan 1, 1999-Dec 31, 2006 | New York City, USA | Restricted to the warm season (Apr to Aug) | 24 Fixed sites; a daily arithmetic mean was calculated and used | HA | TS | GLM with Poisson regression | 131 | Single-pollutant model |
| <6 y | (a) <6 y, | ||||||||
| - Non-ICU admission: 15 185, | - Non-ICU: 1.14 (1.10, 1.19) for 2-d average IQR increase | ||||||||
| - ICU admission: 1141 patients | - ICU: 1.03 (0.91, 1.17) for 2-d average IQR increase | ||||||||
| 6-18y | (b) 6-18 y, | ||||||||
| - Non-ICU admission: 10 332, | - Non-ICU: 1.19 (1.11, 1.27) for 2-d average IQR increase | ||||||||
| - ICU admission: 994 patients | - ICU: 1.26 (1.10, 1.44) for 2-d average IQR increase | ||||||||
| Two-pollutant model with O3 | |||||||||
| (a) <6 y, | |||||||||
| - Non-ICU: 1.13 (1.08, 1.18) for 2-d average IQR increase | |||||||||
| - ICU: 1.04 (0.91, 1.19) for 2-d average IQR increase | |||||||||
| (b) 6-18 y, | |||||||||
| - Non-ICU: 1.16 (1.08, 1.23) for 2-d average IQR increase | |||||||||
| - ICU: 1.23 (1.07-1.41) for 2-d average IQR increase | |||||||||
| Strickland et al. (2010) [15] | Aug 1, 1998-Dec 31, 2004 | Atlanta, USA | 5-17 y, 91 386 patients | 11 Fixed sites; a population-weighting average across monitors was calculated and used | ED visits | TS | GLM with Poisson regression | 16.4 (7.4) | Single-pollutant model |
| - Whole period: 1.020 (1.002,1.039) for 3-d average IQR increase | |||||||||
| - Warm season: 1.043 (1.016, 1.070) for 3-d average IQR increase | |||||||||
| - Cold season: 1.005 (0.978, 1.031) for 3-d average IQR increase | |||||||||
| Li et al. (2011) [16] | Jan 1, 2004-Dec 31, 2006 | Detroit, USA | 2-18 y, 7063 patients | 4 Fixed sites; a daily arithmetic mean was calculated and used | ED visits + HA2 | TS and CCD | GAM and conditional logistic regression | 15.0 (7.9) | Single-pollutant model |
| 1.030 (1.001, 1.061) for 5 d average IQR increase in TS | |||||||||
| 1.039 (1.013, 1.066) for 5 d average IQR increase in CCD | |||||||||
| Glad et al. (2012) [17] | Jan 1, 2002-Dec 31, 2005 | Pittsburgh, USA | 0-17 y, 978 patients | 2 Fixed sites; a daily arithmetic mean was calculated and used | ED visits | CCD | Conditional logistic regression | NA | Single-pollutant model |
| 1.012 (0.916, 1.118) for the same-day 10 μg/m3 increase | |||||||||
| Iskandar et al. (2012) [18] | May 15, 2001-Dec 31, 2008 | Copenhagen, Denmark | 0-18 y, 6329 patients | 1 Fixed site; a daily arithmetic mean was calculated and used | HA | CCD | Conditional logistic regression | 10.3 (5.4) | Single-pollutant model |
| 1.09 (1.04, 1.13) for 5-d average IQR increase | |||||||||
| Two-pollutant model with NO2: | |||||||||
| 1.06 (1.02, 1.11) for 5-d average IQR increase | |||||||||
| Winquist et al. (2012) [19] | Jan 1, 2001-Jun 27, 2007 | St. Louis, USA | 0-1 y. | 1 Fixed site; a daily arithmetic mean was calculated and used | ED visits & HA | TS | GLM with Poisson regression | 14.4 (7.5) | Single-pollutant model |
| - ED: 12 236 patients | (a) 0-1 y, | ||||||||
| 2-18 y. | - ED: 1.047 (0.999, 1.097) for 5-d average IQR increase | ||||||||
| - ED: 49 978 patients | (b) 2-18 y, | ||||||||
| - All HA: 7095 patients | - ED: 1.050 (1.021,1.080) for 5-d average IQR increase | ||||||||
| - HA: 1.052 (0.985, 1.123) for 5-d average IQR increase | |||||||||
| Delfino et al. (2014) [20] | Jan 1, 2000-Dec 31, 2008 | California, USA | 0-18 y, 11 390 patients | Subject addresses were geocoded; using a modified, California LINE Source Dispersion Model, version. 4 to estimate pollutants at each residence | ED visits + HA2 | CCD | Conditional logistic regression | - Warm season: 16.0 (9.5) | Single-pollutant model |
| - Cool season: 19.0 (13.8) | - Warm season: 1.079 (1.008, 1.154) for 7-d average IQR increase | ||||||||
| - Cool season: 1.162 (1.076, 1.254) for 7-d average IQR increase | |||||||||
| Gleason et al. (2014) [21] | Jan 1, 2004-Dec 31, 2007 | New Jersey, USA | 3-17 y, 21 854 patients | Subject addresses were geocoded; using 12×12-km grid from the Multi-Scale Air Quality Model to estimate pollutants at each residence | ED visits | CCD | Conditional logistic regression | NA | Single-pollutant model |
| 1.03 (1.02, 1.04) for the same day IQR increase | |||||||||
| Multipollutant model with O3 and other pollens | |||||||||
| 0.99 (0.98, 1.01) for the same day IQR increase | |||||||||
| Hua et al. (2014) [32] | Jan 1, 2007-Jul 31, 2012 | Shanghai, China | 0-14 y, 114 673 patients | 1 Fixed site; a daily arithmetic mean was calculated and used | HA | TS | Polynomial distributed lag model | 40.9 (27.7) | Single-pollutant model |
| 1.04 (1.02, 1.05) for IQR increase with a maximum lag of 3 d | |||||||||
| 1.06 (1.05, 1.08) for IQR increase with a maximum lag of 5 d | |||||||||
| Multipollutant model with NO2 and SO2 | |||||||||
| 1.03 (1.02, 1.05) for IQR increase with a maximum lag of 3 d | |||||||||
| 1.06 (1.04, 1.08) for IQR increase with a maximum lag of 5 d | |||||||||
| Strickland et al. (2014) [22] | Jan 1, 2002-Jun 30, 2010 | Atlanta, USA | 2-16 y, 109 758 patients | 6 Fixed sites; a population-weighting average across monitors calculated and used | ED visits | TS | GLM with Poisson regression | 13.3 (5.4) | Single-pollutant model |
| 1.032 (1.019, 1.044) for 3-d average IQR increase | |||||||||
| Two-pollutant model with O3 | |||||||||
| 1.022 (1.009, 1.035) for 3-d average IQR increase | |||||||||
| Wendt et al. (2014) [23] | Jan 1, 2005-Dec 31, 2007 | Boston, USA | 0-17 y | 3 Fixed sites; a daily arithmetic mean was calculated and used | HA | CCD | Conditional logistic regression | 15.0 (6.0) | Single-pollutant model |
| - May to Oct: 6061 patients | - May to Oct: 1.10 (1.03, 1.17) for 6-d average IQR increase | ||||||||
| - Nov to Apr: 7894 patients | - Nov to April: 1.06 (1.00, 1.14) for 6-d average IQR increase | ||||||||
| Two-pollutant model with NO2 | |||||||||
| - May to Oct: 1.13 (1.04, 1.24) for 6-d average IQR increase | |||||||||
| - Nov to Apr: 1.00 (0.93, 1.07) for 6-d average IQR increase | |||||||||
| Byers et al. (2016) [24] | Jan 1, 2007-Dec 31, 2011 | Indianapolis, USA | 5-17 y, 33 981 patients | 3 Fixed sites; a population-weighting average across monitors calculated and used | ED visits | TS | GLM with Poisson regression | 13.6 (7.1) | Single-pollutant model |
| - All seasons: 1.007 (0.986, 1.029) for 3-d average IQR increase | |||||||||
| - Apr to Sep: 0.985 (0.934, 1.040) for 3-d average IQR increase | |||||||||
| - Oct to Mar: 0.976 (0.930, 1.025) for 3-d average IQR increase | |||||||||
| Gleason et al. (2015) [25] | Jan 1, 2004-Dec 31, 2007 | Newark, USA | 3-17 y, 3675 patients | Subject addresses were geocoded; using grid from the Multi-Scale Air Quality Model to estimate pollutants at each residence | ED visits | TS and CCD | GLM and conditional logistic regression | NA | Single-pollutant model |
| 1.00 (0.96, 1.05) for 3-d average IQR increase in TS | |||||||||
| 1.00 (0.96, 1.04) for 3-d average IQR increase in CCD | |||||||||
| Multipollutant model with O3 and other pollens | |||||||||
| 0.93 (0.89, 0.98) for 3-d average IQR increase in TS | |||||||||
| 0.95 (0.91, 1.00) for 3-d average IQR increase in CCD | |||||||||
| Strickland et al. (2015) [26] | Jan 1, 2002-Jun 30, 2010 | Georgia, USA | 2-18 y, 189 816 patients | Subject addresses were geocoded; using a two-stage model that includes land use parameters and satellite aerosol optical depth measurements at 1-km resolution to estimate pollutants | ED visits | CCD | Conditional logistic regression | 12.91 | Single-pollutant model |
| 1.013 (1.003, 1.023) for the same day 10 μg/m3 increase | |||||||||
| Alhanti et al. (2016) [27] | Jan 1, 2006-Dec 31, 2009 | Dallas, USA | 0-4 y, mean daily counts: 16.91 patients | All available monitors; the monitoring data were first spatially interpolated across the study’s geographic domain and then a population-weighted average across monitors calculated and used | ED visits | TS | GLM with Poisson regression | 11.1 (4.7) | Single-pollutant model |
| 5-18 y, mean daily counts: 25.75 patients | 0-4 y, 0.98 (0.94, 1.02) for 3-d average IQR increase | ||||||||
| 5-18 y, 0.99 (0.95, 1.03) for 3-d average IQR increase | |||||||||
| Weichenthal et al. (2016) [28] | Jan 1, 2004-Dec 31, 2011 | Ontario, Canada | Total; 127 836 patients, | Fixed site in Ontario which is part of Canada’s National Air Pollution Surveillance network; a daily arithmetic mean was calculated and used | ED visits | CCD | Conditional logistic regression | 7.1 (6.3) | Single-pollutant model |
| <9y, NA | 1.072 (1.042, 1.100) for 3-d average IQR increase |
Ref, reference number; HA, hospital admission; ED, emergency department; GLM, generalized linear model; GAM, generalized additive model; NA, not available; IQR, interquartile range; TS, time series; CCD, case-crossover design; PM, particulate matter; SD, standard devaition; CI, confidence interval; ICU, intensive care unit; CO, carbon monoxide; SO2, sulfur dioxide; NO2, nitrogen dioxide; O3, ozone.
1 Median value of the daily PM2.5 distribution during the entire study period. This study doesn’t present the arithmetic mean of PM2.5.
2 The authors regarded asthma morbidity as hospital encounters which counted both HA and ED visits.
| No. of study (no. of estimate) | RR (95% CIs)1 | I2 (%) | |
|---|---|---|---|
| Age2 | |||
| < 5 | 7 (9) | 1.044 (1.017, 1.071) | 81.9 |
| 5-18 | 12 (15) | 1.027 (1.011, 1.043) | 76.8 |
| Outcome | |||
| HA | 10 (15) | 1.048 (1.029, 1.067) | 77.7 |
| ED visits | 15 (17) | 1.027 (1.011, 1.044) | 79.5 |
| Season | |||
| Cold | 7 (8) | 1.015 (0.994, 1.037) | 57.1 |
| Warm | 9 (11) | 1.085 (1.051, 1.119) | 94.8 |
| Study design | |||
| TS | 15 (19) | 1.028 (1.015, 1.041) | 76.9 |
| CCD | 13 (17) | 1.051 (1.020, 1.084) | 96.6 |
| Area | |||
| North America | 14 (19) | 1.047 (1.019, 1.076) | 96.1 |
| Europe | 8 (11) | 1.075 (1.030, 1.123) | 65.9 |
| China | 3 (3) | 1.019 (1.013, 1.025) | 0.0 |
| Multipollutant model | |||
| No | 25 (33) | 1.054 (1.037, 1.071) | 96.0 |
| Yes | 13 (18) | 1.040 (1.022, 1.057) | 83.1 |
| Time lag (d) | |||
| 0 (same day) | 12 (14) | 1.018 (1.005, 1.028) | 60.9 |
| 1 | 11 (13) | 1.018 (1.005, 1.030) | 59.6 |
| 2 | 8 (8) | 1.002 (0.984, 1.021) | 84.6 |
| 3 | 10 (11) | 1.030 (1.015, 1.045) | 66.6 |
| 4 | 4 (4) | 1.016 (0.969, 1.065) | 83.1 |
| 5 | 5 (6) | 1.019 (0.975, 1.065) | 93.5 |
| Average | |||
| 2 | 3 (7) | 1.065 (1.020, 1.113) | 81.7 |
| 3 | 11 (15) | 1.019 (1.006, 1.033) | 82.2 |
| 5 | 10 (14) | 1.025 (1.007, 1.043) | 77.4 |
| 6 | 3 (5) | 1.029 (0.938, 1.129) | 69.9 |
- 1. Vallero DA. Fundamentals of air pollution. 5th ed. Amsterdam: Academic Press; 2014. p. 48
- 2. Pope CA 3rd, Burnett RT, Krewski D, Jerrett M, Shi Y, Calle EE, et al. Cardiovascular mortality and exposure to airborne fine particulate matter and cigarette smoke: shape of the exposure-response relationship. Circulation 2009;120(11):941-948ArticlePubMed
- 3. Lepeule J, Laden F, Dockery D, Schwartz J. Chronic exposure to fine particles and mortality: an extended follow-up of the Harvard Six Cities study from 1974 to 2009. Environ Health Perspect 2012;120(7):965-970ArticlePubMedPMC
- 4. Kwon HJ. Epidemiologic study of allergic diseases in Korean children. Cheongju: Korea Centers for Disease Control and Prevention; 2011. p. 23-24 (Korean)
- 5. Zheng XY, Ding H, Jiang LN, Chen SW, Zheng JP, Qiu M, et al. Association between air pollutants and asthma emergency room visits and hospital admissions in time series studies: a systematic review and meta-analysis. PLoS One 2015;10(9):e0138146Article
- 6. Fan J, Li S, Fan C, Bai Z, Yang K. The impact of PM2.5 on asthma emergency department visits: a systematic review and meta-analysis. Environ Sci Pollut Res Int 2016;23(1):843-850ArticlePubMedPDF
- 7. Norris G, YoungPong SN, Koenig JQ, Larson TV, Sheppard L, Stout JW. An association between fine particles and asthma emergency department visits for children in Seattle. Environ Health Perspect 1999;107(6):489-493Article
- 8. Lin M, Chen Y, Burnett RT, Villeneuve PJ, Krewski D. The influence of ambient coarse particulate matter on asthma hospitalization in children: case-crossover and time-series analyses. Environ Health Perspect 2002;110(6):575-581ArticlePubMedPMC
- 9. Villeneuve PJ, Chen L, Rowe BH, Coates F. Outdoor air pollution and emergency department visits for asthma among children and adults: a case-crossover study in northern Alberta, Canada. Environ Health 2007;6: 40ArticlePubMedPMCPDF
- 10. Andersen ZJ, Wahlin P, Raaschou-Nielsen O, Ketzel M, Scheike T, Loft S. Size distribution and total number concentration of ultrafine and accumulation mode particles and hospital admissions in children and the elderly in Copenhagen, Denmark. Occup Environ Med 2008;65(7):458-466ArticlePubMed
- 11. Halonen J, Lanki T, Yli-Tuomi T, Kulmala M, Tiittanen P, Pekkanen J. Urban air pollution, and asthma and COPD hospital emergency room visits. Thorax 2008;63(7):635-641ArticlePubMed
- 12. Tecer LH, Alagha O, Karaca F, Tuncel G, Eldes N. Particulate matter (PM(2.5), PM(10-2.5), and PM(10)) and children’s hospital admissions for asthma and respiratory diseases: a bidirectional case-crossover study. J Toxicol Environ Health A 2008;71(8):512-520ArticlePubMed
- 13. Halonen JI, Lanki T, Tiittanen P, Niemi JV, Loh M, Pekkanen J. Ozone and cause-specific cardiorespiratory morbidity and mortality. J Epidemiol Community Health 2010;64(9):814-820ArticlePubMed
- 14. Silverman RA, Ito K. Age-related association of fine particles and ozone with severe acute asthma in New York City. J Allergy Clin Immunol 2010;125(2):367-373ArticlePubMed
- 15. Strickland MJ, Darrow LA, Klein M, Flanders WD, Sarnat JA, Waller LA, et al. Short-term associations between ambient air pollutants and pediatric asthma emergency department visits. Am J Respir Crit Care Med 2010;182(3):307-316ArticlePubMedPMC
- 16. Li S, Batterman S, Wasilevich E, Wahl R, Wirth J, Su FC, et al. Association of daily asthma emergency department visits and hospital admissions with ambient air pollutants among the pediatric Medicaid population in Detroit: time-series and time-stratified case-crossover analyses with threshold effects. Environ Res 2011;111(8):1137-1147ArticlePubMed
- 17. Glad JA, Brink LL, Talbott EO, Lee PC, Xu X, Saul M, et al. The relationship of ambient ozone and PM(2.5) levels and asthma emergency department visits: possible influence of gender and ethnicity. Arch Environ Occup Health 2012;67(2):103-108ArticlePubMed
- 18. Iskandar A, Andersen ZJ, Bønnelykke K, Ellermann T, Andersen KK, Bisgaard H. Coarse and fine particles but not ultrafine particles in urban air trigger hospital admission for asthma in children. Thorax 2012;67(3):252-257ArticlePubMed
- 19. Winquist A, Klein M, Tolbert P, Flanders WD, Hess J, Sarnat SE. Comparison of emergency department and hospital admissions data for air pollution time-series studies. Environ Health 2012;11: 70ArticlePubMedPMCPDF
- 20. Delfino RJ, Wu J, Tjoa T, Gullesserian SK, Nickerson B, Gillen DL. Asthma morbidity and ambient air pollution: effect modification by residential traffic-related air pollution. Epidemiology 2014;25(1):48-57ArticlePubMed
- 21. Gleason JA, Bielory L, Fagliano JA. Associations between ozone, PM2.5, and four pollen types on emergency department pediatric asthma events during the warm season in New Jersey: a case-crossover study. Environ Res 2014;132: 421-429ArticlePubMed
- 22. Strickland MJ, Klein M, Flanders WD, Chang HH, Mulholland JA, Tolbert PE, et al. Modification of the effect of ambient air pollution on pediatric asthma emergency visits: susceptible subpopulations. Epidemiology 2014;25(6):843-850ArticlePubMedPMC
- 23. Wendt JK, Symanski E, Stock TH, Chan W, Du XL. Association of short-term increases in ambient air pollution and timing of initial asthma diagnosis among Medicaid-enrolled children in a metropolitan area. Environ Res 2014;131: 50-58ArticlePubMedPMC
- 24. Byers N, Ritchey M, Vaidyanathan A, Brandt AJ, Yip F. Short-term effects of ambient air pollutants on asthma-related emergency department visits in Indianapolis, Indiana, 2007-2011. J Asthma 2016;53(3):245-252ArticlePubMed
- 25. Gleason JA, Fagliano JA. Associations of daily pediatric asthma emergency department visits with air pollution in Newark, NJ: utilizing time-series and case-crossover study designs. J Asthma 2015;52(8):815-822PubMed
- 26. Strickland MJ, Hao H, Hu X, Chang HH, Darrow LA, Liu Y. Pediatric emergency visits and short-term changes in PM2.5 concentrations in the U.S. State of Georgia. Environ Health Perspect 2016;124(5):690-696ArticlePubMed
- 27. Alhanti BA, Chang HH, Winquist A, Mulholland JA, Darrow LA, Sarnat SE. Ambient air pollution and emergency department visits for asthma: a multi-city assessment of effect modification by age. J Expo Sci Environ Epidemiol 2016;26(2):180-188ArticlePubMed
- 28. Weichenthal SA, Lavigne E, Evans GJ, Godri Pollitt KJ, Burnett RT. PM2.5 and emergency room visits for respiratory illness: effect modification by oxidative potential. Am J Respir Crit Care Med 2016. doi: http://dx.doi.org/10.1164/rccm.201512-2434OC
- 29. Lee SL, Wong WH, Lau YL. Association between air pollution and asthma admission among children in Hong Kong. Clin Exp Allergy 2006;36(9):1138-1146ArticlePubMedPMC
- 30. Ko FW, Tam W, Wong TW, Lai CK, Wong GW, Leung TF, et al. Effects of air pollution on asthma hospitalization rates in different age groups in Hong Kong. Clin Exp Allergy 2007;37(9):1312-1319ArticlePubMed
- 31. Jalaludin B, Khalaj B, Sheppeard V, Morgan G. Air pollution and ED visits for asthma in Australian children: a case-cross-over analysis. Int Arch Occup Environ Health 2008;81(8):967-974ArticlePubMed
- 32. Hua J, Yin Y, Peng L, Du L, Geng F, Zhu L. Acute effects of black carbon and PM2.5 on children asthma admissions: a time-series study in a Chinese city. Sci Total Environ 2014;481: 433-438ArticlePubMed
- 33. DerSimonian R, Laird N. Meta-analysis in clinical trials. Control Clin Trials 1986;7(3):177-188ArticlePubMed
- 34. Kim SY, Park JE, Seo HJ, Lee YJ, Jang BH, Son HJ, et al. NECA’s guidance for undertaking systematic reviews and meta-analyses for intervention. Seoul: National Evidence-based Healthcare Collaborating Agency; 2011. p. 141-142 (Korean)
- 35. Anderson HR, Atkinson RW, Peacock JL, Marston L, Konstantinou K. Meta-analysis of time-series studies and panel studies of particulate matter (PM) and ozone (O3): report of a WHO task group. Copenhagen: WHO Regional Office for Europe; 2004. p. 8
- 36. Ruana Y, Liangb R, Liana H, Zhaoa X, Liua X, Fan Z. Endothelial function and short-term exposure to particulate matter: a systematic review and meta-analysis. J Environ Anal Chem 2015;2(5):159
- 37. Egger M, Davey Smith G, Schneider M, Minder C. Bias in meta-analysis detected by a simple, graphical test. BMJ 1997;315(7109):629-634ArticlePubMedPMC
- 38. World Health Organization Regional Office for Europe. Effects of air pollution on children’s health and development: a review of the evidence. Copenhagen: World Health Organization Regional Office for Europe; 2005. p. 12
- 39. Gavett SH, Koren HS. The role of particulate matter in exacerbation of atopic asthma. Int Arch Allergy Immunol 2001;124(1-3):109-112ArticlePubMed
- 40. Salam MT, Islam T, Gauderman WJ, Gilliland FD. Roles of arginase variants, atopy, and ozone in childhood asthma. J Allergy Clin Immunol 2009;123(3):596-602ArticlePubMedPMC
- 41. North ML, Khanna N, Marsden PA, Grasemann H, Scott JA. Functionally important role for arginase 1 in the airway hyperresponsiveness of asthma. Am J Physiol Lung Cell Mol Physiol 2009;296(6):L911-L920ArticlePubMed
- 42. North ML, Amatullah H, Khanna N, Urch B, Grasemann H, Silverman F, et al. Augmentation of arginase 1 expression by exposure to air pollution exacerbates the airways hyperresponsiveness in murine models of asthma. Respir Res 2011;12: 19ArticlePubMedPMCPDF
- 43. Li H, Romieu I, Sienra-Monge JJ, Ramirez-Aguilar M, Estela Del Rio-Navarro B, Kistner EO, et al. Genetic polymorphisms in arginase I and II and childhood asthma and atopy. J Allergy Clin Immunol 2006;117(1):119-126ArticlePubMed
- 44. Sarnat SE, Coull BA, Schwartz J, Gold DR, Suh HH. Factors affecting the association between ambient concentrations and personal exposures to particles and gases. Environ Health Perspect 2006;114(5):649-654ArticlePubMed
- 45. Lee PK, Brook JR, Dabek-Zlotorzynska E, Mabury SA. Identification of the major sources contributing to PM2.5 observed in Toronto. Environ Sci Technol 2003;37(21):4831-4840ArticlePubMed
- 46. Perrone MG, Gualtieri M, Ferrero L, Lo Porto C, Udisti R, Bolzacchini E, et al. Seasonal variations in chemical composition and in vitro biological effects of fine PM from Milan. Chemosphere 2010;78(11):1368-1377ArticlePubMed
- 47. Li X, Wang Y, Guo X, Wang Y. Seasonal variation and source apportionment of organic and inorganic compounds in PM2.5 and PM10 particulates in Beijing, China. J Environ Sci (China) 2013;25(4):741-750ArticlePubMed
- 48. Pui DY, Chen SC, Zuo Z. PM2.5 in China: measurements, sources, visibility and health effects, and mitigation. Particuology 2014;13: 1-26Article
- 49. Lu F, Xu D, Cheng Y, Dong S, Guo C, Jiang X, et al. Systematic review and meta-analysis of the adverse health effects of ambient PM2.5 and PM10 pollution in the Chinese population. Environ Res 2015;136: 196-204ArticlePubMed
- 50. Franklin M, Zeka A, Schwartz J. Association between PM2.5 and all-cause and specific-cause mortality in 27 US communities. J Expo Sci Environ Epidemiol 2007;17(3):279-287ArticlePubMed
- 51. Wong GW, Chow CM. Childhood asthma epidemiology: insights from comparative studies of rural and urban populations. Pediatr Pulmonol 2008;43(2):107-116ArticlePubMed
- 52. Schröder PC, Li J, Wong GW, Schaub B. The rural-urban enigma of allergy: what can we learn from studies around the world? Pediatr Allergy Immunol 2015;26(2):95-102ArticlePubMed
- 53. Seong SC, Son MS. National health insurance statistical year-book 2014. Seoul: Korean Health Insurance Review & Assessment Service; 2015. p. 470-473 (Korean)
REFERENCES
Figure & Data
References
Citations

- Do respiratory virus infections modify associations of asthma exacerbation with aeroallergens or fine particulate matter? A time series study in Philadelphia PA
Wanyu Huang, Leah H. Schinasi, Chén C. Kenyon, Amy H. Auchincloss, Kari Moore, Steven Melly, Lucy F. Robinson, Christopher B. Forrest, Anneclaire J. De Roos
International Journal of Environmental Health Research.2024; : 1. CrossRef - Residential surrounding greenness and the incidence of childhood asthma: Findings from a population-based cohort in Ontario, Canada
Razieh Mansouri, Eric Lavigne, Robert Talarico, Audrey Smargiassi, Laura A. Rodriguez-Villamizar, Paul J. Villeneuve
Environmental Research.2024; 249: 118316. CrossRef - Contemporary Concise Review 2023: Environmental and occupational lung diseases
Kazuhiro Yatera, Chinatsu Nishida
Respirology.2024; 29(7): 574. CrossRef - Short-term exposure to fine particulate matter exposure impairs innate immune and inflammatory responses to a pathogen stimulus: A functional study in the zebrafish model
Marco Cafora, Sabrina Rovelli, Andrea Cattaneo, Anna Pistocchi, Luca Ferrari
Environmental Pollution.2024; 348: 123841. CrossRef - Association of Short-Term Increases in Ambient Fine Particulate Matter With Hospitalization for Asthma or COPD During Wildfire Season and Other Time Periods
Benjamin D. Horne, Mary M. Johnson, Denitza P. Blagev, Francois Haddad, Kirk U. Knowlton, Daniel Bride, Tami L. Bair, Elizabeth A. Joy, Kari C. Nadeau
CHEST Pulmonary.2024; 2(2): 100053. CrossRef - Risk Factors for Acute Asthma Exacerbations in Adults With Mild Asthma
Wansu Chen, Eric J. Puttock, Michael Schatz, William Crawford, William M. Vollmer, Fagen Xie, Stanley Xu, Eva Lustigova, Robert S. Zeiger
The Journal of Allergy and Clinical Immunology: In Practice.2024;[Epub] CrossRef - The Role of Neighborhood Air Pollution in Disparate Racial and Ethnic Asthma Acute Care Use
Sarah E. Chambliss, Elizabeth C. Matsui, Rebecca A. Zárate, Corwin M. Zigler
American Journal of Respiratory and Critical Care Medicine.2024; 210(2): 178. CrossRef - Acute Effects of Ambient Air Pollution on Asthma Emergency Department Visits in Ten U.S. States
Jianzhao Bi, Rohan R. D’Souza, Shannon Moss, Niru Senthilkumar, Armistead G. Russell, Noah C. Scovronick, Howard H. Chang, Stefanie Ebelt
Environmental Health Perspectives.2023;[Epub] CrossRef - The Impact of Climate Change on Asthma and Allergic-Immunologic Disease
Grace Kelly, Osatohamwen I. Idubor, Sophie Binney, Paul J. Schramm, Maria C. Mirabelli, Joy Hsu
Current Allergy and Asthma Reports.2023; 23(8): 453. CrossRef - eHealth Technologies for Monitoring Pediatric Asthma at Home: Scoping Review
Mattiènne R van der Kamp, Vera S Hengeveld, Marjolein G J Brusse-Keizer, Boony J Thio, Monique Tabak
Journal of Medical Internet Research.2023; 25: e45896. CrossRef - Environmental Influences and Allergic Diseases in the Asia-Pacific Region: What Will Happen in Next 30 Years?
Yuhan Xing, Gary Wing-Kin Wong
Allergy, Asthma & Immunology Research.2022; 14(1): 21. CrossRef - Forecasting the Effects of Real-Time Indoor PM2.5 on Peak Expiratory Flow Rates (PEFR) of Asthmatic Children in Korea: A Deep Learning Approach
Jiyoung Woo, Ji-Hyun Lee, Yeonjin Kim, Guillaume Rudasingwa, Dae Hyun Lim, Sungroul Kim
IEEE Access.2022; 10: 19391. CrossRef - The Impact of Ambient Environmental and Occupational Pollution on Respiratory Diseases
Chinatsu Nishida, Kazuhiro Yatera
International Journal of Environmental Research and Public Health.2022; 19(5): 2788. CrossRef - Indoor air pollution effects on pediatric asthma are submicron aerosol particle–dependent
Izabele Juskiene, Nina Prokopciuk, Ulrich Franck, Algirdas Valiulis, Vaidotas Valskys, Vitalija Mesceriakova, Violeta Kvedariene, Indre Valiulyte, Edita Poluzioroviene, Ingrida Sauliene, Arunas Valiulis
European Journal of Pediatrics.2022; 181(6): 2469. CrossRef - PM2.5 Exposure and Asthma Development: The Key Role of Oxidative Stress
Kaimeng Liu, Shucheng Hua, Lei Song, Alin Ciobica
Oxidative Medicine and Cellular Longevity.2022; 2022: 1. CrossRef - Outdoor air quality and human health: An overview of reviews of observational studies
Georgios Markozannes, Katerina Pantavou, Evangelos C. Rizos, Ourania Α. Sindosi, Christos Tagkas, Maike Seyfried, Ian J. Saldanha, Nikos Hatzianastassiou, Georgios K. Nikolopoulos, Evangelia Ntzani
Environmental Pollution.2022; 306: 119309. CrossRef - New Homogeneous Spatial Areas Identified Using Case-Crossover Spatial Lag Grid Differences between Aerosol Optical Depth-PM2.5 and Respiratory-Cardiovascular Emergency Department Visits and Hospitalizations
John T. Braggio, Eric S. Hall, Stephanie A. Weber, Amy K. Huff
Atmosphere.2022; 13(5): 719. CrossRef - Increasing Prevalence of Allergic Disease and Its Impact on Current Practice
Sofia E. Edwards-Salmon, Shree Lakshmi Padmanabhan, Merin Kuruvilla, Joshua M. Levy
Current Otorhinolaryngology Reports.2022; 10(3): 278. CrossRef - The Correlation of PM2.5 Exposure with Acute Attack and Steroid Sensitivity in Asthma
Jingjing Luo, Han Liu, Shucheng Hua, Lei Song, Jianfeng Wang
BioMed Research International.2022; 2022: 1. CrossRef - Application of high-resolution metabolomics to identify biological pathways perturbed by traffic-related air pollution
Zhenjiang Li, Donghai Liang, Dongni Ye, Howard H. Chang, Thomas R. Ziegler, Dean P. Jones, Stefanie T. Ebelt
Environmental Research.2021; 193: 110506. CrossRef - Review of epidemiological studies on air pollution and health effects in children
Jong-Tae Lee
Clinical and Experimental Pediatrics.2021; 64(1): 3. CrossRef - The Effect of Coal-Fired Power Plant Closures on Emergency Department Visits for Asthma-Related Conditions Among 0- to 4-Year-Old Children in Chicago, 2009–2017
Sarah Komisarow, Emily L. Pakhtigian
American Journal of Public Health.2021; 111(5): 881. CrossRef - The clear and persistent impact of air pollution on chronic respiratory diseases: a call for interventions
Isabella Annesi-Maesano, Francesco Forastiere, John Balmes, Erika Garcia, Jack Harkema, Stephen Holgate, Frank Kelly, Haneen Khreis, Barbara Hoffmann, Cara Nichole Maesano, Rob McConnell, David Peden, Kent Pinkerton, Tamara Schikowski, George Thurston, La
European Respiratory Journal.2021; 57(3): 2002981. CrossRef - The Multiple Benefits of Removing Major Outdoor Air Pollution Point Sources
Derek G. Shendell
American Journal of Public Health.2021; 111(5): 770. CrossRef - Assessing the Distribution of Air Pollution Health Risks within Cities: A Neighborhood-Scale Analysis Leveraging High-Resolution Data Sets in the Bay Area, California
Veronica A. Southerland, Susan C. Anenberg, Maria Harris, Joshua Apte, Perry Hystad, Aaron van Donkelaar, Randall V. Martin, Matt Beyers, Ananya Roy
Environmental Health Perspectives.2021;[Epub] CrossRef - The role of the environment in shaping the trends of childhood asthma – An Asian perspective
Agnes S. Y. Leung, Elizabeth Huiwen Tham, Jing Li, Punchama Pacharn, Takumi Takizawa, Eun Lee, Yuhan Xing, Ting‐Fan Leung, Soo‐Jong Hong, Gary W. K. Wong, Ömer Kalaycı
Pediatric Allergy and Immunology.2021; 32(6): 1152. CrossRef - Impact of a pollution breach at a coke oven factory on asthma control in nearby vulnerable adults
Brandy M. Byrwa-Hill, Albert A. Presto, Sally Wenzel, James P. Fabisiak
Journal of Allergy and Clinical Immunology.2021; 148(1): 225. CrossRef - Intervention of Particulate Matter: What Can We Do for Asthmatic Patients?
Kangmo Ahn
Allergy, Asthma & Immunology Research.2021; 13(5): 677. CrossRef - Quantifying the Health Benefits of Face Masks and Respirators to Mitigate Exposure to Severe Air Pollution
John K. Kodros, Katelyn O’Dell, Jonathan M. Samet, Christian L’Orange, Jeffrey R. Pierce, John Volckens
GeoHealth.2021;[Epub] CrossRef - Combinations of Epidemiological and Experimental Studies in Air Pollution Research: A Narrative Review
Hannah Weisenberg, Tianyu Zhao, Joachim Heinrich
International Journal of Environmental Research and Public Health.2020; 17(2): 385. CrossRef - A risk-based model to assess environmental justice and coronary heart disease burden from traffic-related air pollutants
James P. Fabisiak, Erica M. Jackson, LuAnn L. Brink, Albert A. Presto
Environmental Health.2020;[Epub] CrossRef - Impact of Air Pollution on Asthma Outcomes
Angelica I. Tiotiu, Plamena Novakova, Denislava Nedeva, Herberto Jose Chong-Neto, Silviya Novakova, Paschalis Steiropoulos, Krzysztof Kowal
International Journal of Environmental Research and Public Health.2020; 17(17): 6212. CrossRef - Impact of Air Pollution and Trans-Boundary Haze on Nation-Wide Emergency Department Visits and Hospital Admissions in Singapore
Sze Ling Chan, Andrew FW Ho, Huicong Ding, Nan Liu, Arul Earnest, Mariko S Koh, Jolyn ST Chuah, Zheng Yi Lau, Kelvin Bryan Tan, Huili Zheng, Geoffrey G Morgan, Marcus EH Ong
Annals of the Academy of Medicine, Singapore.2020; 49(2): 78. CrossRef - High Temperatures and Kidney Disease Morbidity: A Systematic Review and Meta-analysis
Woo-Seok Lee, Woo-Sung Kim, Youn-Hee Lim, Yun-Chul Hong
Journal of Preventive Medicine and Public Health.2019; 52(1): 1. CrossRef - Identifying and characterizing the effects of calendar and environmental conditions on pediatric admissions in Shanghai
Guang-jun Yu, Jian-lei Gu, Wen-bin Cui, Jian-ping Jiang, Yang Wang, Georgi Z. Genchev, Ting Lu, Hui Lu
Journal of Big Data.2019;[Epub] CrossRef - Air Pollution, Asthma, and Sleep Apnea: New Epidemiological Links?
Gökhan M. Mutlu, Yüksel Peker
Annals of the American Thoracic Society.2019; 16(3): 307. CrossRef - Using Syndromic Surveillance to Evaluate the Respiratory Effects of Fine Particulate Matter
Christina H. Fuller, Douglas Roblin, Jordan Jones
Annals of the American Thoracic Society.2019; 16(7): 930. CrossRef - Ambient air pollution is associated with pediatric pneumonia: a time-stratified case–crossover study in an urban area
Chi-Yung Cheng, Shih-Yu Cheng, Chien-Chih Chen, Hsiu-Yung Pan, Kuan-Han Wu, Fu-Jen Cheng
Environmental Health.2019;[Epub] CrossRef - The Effects of Short-Term and Very Short-Term Particulate Matter Exposure on Asthma-Related Hospital Visits: National Health Insurance Data
Dae Jin Song, Sun Hee Choi, Woo-Jung Song, Kyung Hee Park, Young-Koo Jee, Sang-Heon Cho, Dae Hyun Lim
Yonsei Medical Journal.2019; 60(10): 952. CrossRef - Association between fire smoke fine particulate matter and asthma-related outcomes: Systematic review and meta-analysis
Nicolas Borchers Arriagada, Joshua A. Horsley, Andrew J. Palmer, Geoffrey G. Morgan, Rachel Tham, Fay H. Johnston
Environmental Research.2019; 179: 108777. CrossRef - Review of the effect of air pollution exposure from industrial point sources on asthma-related effects in childhood
Stéphane Buteau, Xiaohui Geng, Remi Labelle, Audrey Smargiassi
Environmental Epidemiology.2019; 3(6): e077. CrossRef - Viruses and non-allergen environmental triggers in asthma
Florence Chau-Etchepare, Joshua L Hoerger, Brooks T Kuhn, Amir A Zeki, Angela Haczku, Samuel Louie, Nicholas J Kenyon, Cristina E Davis, Michael Schivo
Journal of Investigative Medicine.2019; 67(7): 1029. CrossRef - A Review of Airborne Particulate Matter Effects on Young Children’s Respiratory Symptoms and Diseases
Hai-Ying Liu, Daniel Dunea, Stefania Iordache, Alin Pohoata
Atmosphere.2018; 9(4): 150. CrossRef - The association of ambient PM2.5 with school absence and symptoms in schoolchildren: a panel study
Yi Zhang, Liangliang Cui, Dandan Xu, Mike Z. He, Jingwen Zhou, Lianyu Han, Xinwei Li, Tiantian Li
Pediatric Research.2018; 84(1): 28. CrossRef - Changes in Accident & Emergency Visits and Return Visits in Relation to the Enforcement of Daylight Saving Time and Photoperiod
Elena Ferrazzi, Chiara Romualdi, Michele Ocello, Giovanni Frighetto, Matteo Turco, Stefania Vigolo, Fabrizio Fabris, Paolo Angeli, Gianna Vettore, Rodolfo Costa, Sara Montagnese
Journal of Biological Rhythms.2018; 33(5): 555. CrossRef - Exposure to traffic-related air pollution and acute bronchitis in children: season and age as modifiers
Lijun Bai, Xi Su, Desheng Zhao, Yanwu Zhang, Qiang Cheng, Heng Zhang, Shusi Wang, Mingyu Xie, Hong Su
Journal of Epidemiology and Community Health.2018; 72(5): 426. CrossRef - Estimates of the Global Burden of Ambient PM2.5, Ozone, and NO2 on Asthma Incidence and Emergency Room Visits
Susan C. Anenberg, Daven K. Henze, Veronica Tinney, Patrick L. Kinney, William Raich, Neal Fann, Chris S. Malley, Henry Roman, Lok Lamsal, Bryan Duncan, Randall V. Martin, Aaron van Donkelaar, Michael Brauer, Ruth Doherty, Jan Eiof Jonson, Yanko Davila, K
Environmental Health Perspectives.2018;[Epub] CrossRef - Trends in the Prevalence of Childhood Asthma in Seoul Metropolitan City, Korea: The Seoul Atopy ∙ Asthma-friendly School Project
Yong Min Cho, Chea-Bong Kim, Kyung Nam Yeon, Eun Sun Lee, KyooSang Kim
Journal of Preventive Medicine and Public Health.2018; 51(6): 275. CrossRef - Emergency room visits for respiratory diseases associated with ambient fine particulate matter in Taiwan in 2012: A population-based study
Su-Lun Hwang, Yu-Ching Lin, Su-Er Guo, Miao-Ching Chi, Chiang-Ting Chou, Chieh-Mo Lin
Atmospheric Pollution Research.2017; 8(3): 465. CrossRef - Effects of fine particulate matter and its constituents on emergency room visits for asthma in southern Taiwan during 2008–2010: a population-based study
Su-Lun Hwang, Yu-Ching Lin, Chieh-Mo Lin, Kuang-Yu Hsiao
Environmental Science and Pollution Research.2017; 24(17): 15012. CrossRef - Relationship between emergency care utilization, ambient temperature, and the pollution standard index in Taiwan
Ching-hui Tseng, Li-Chin Lu, Shao-Hwan Lan, Yen-Ping Hsieh, Shou-Jen Lan
International Journal of Environmental Health Research.2017; 27(5): 344. CrossRef - Impact of respiratory infections, outdoor pollen, and socioeconomic status on associations between air pollutants and pediatric asthma hospital admissions
Julie E. Goodman, Christine T. Loftus, Xiaobin Liu, Ke Zu, Alexander Larcombe
PLOS ONE.2017; 12(7): e0180522. CrossRef
 KSPM
KSPM

 PubReader
PubReader ePub Link
ePub Link Cite
Cite