Articles
- Page Path
- HOME > J Prev Med Public Health > Volume 53(1); 2020 > Article
-
Original Article
Effects of Comorbid Sleep Disorders on Cardiovascular Complications of Hypertension Among Patients With Newly-diagnosed Hypertension: An Analysis of the Korean National Health Insurance Service-National Sample Cohort -
Jeongmook Kang1
 , Yoon-Hyung Park1
, Yoon-Hyung Park1 , Kwang Ik Yang2
, Kwang Ik Yang2 , Jose Rene Bagani Cruz1
, Jose Rene Bagani Cruz1 , Young Hwangbo1
, Young Hwangbo1
-
Journal of Preventive Medicine and Public Health 2020;53(1):37-44.
DOI: https://doi.org/10.3961/jpmph.19.248
Published online: November 6, 2019
1Department of Preventive Medicine, Soonchunhyang University College of Medicine, Cheonan, Korea
2Sleep Disorders Center, Department of Neurology, Soonchunhyang University College of Medicine, Cheonan Hospital, Cheonan, Korea
- Corresponding author: Young Hwangbo, MD, PhD Department of Preventive Medicine, Soonchunhyang University College of Medicine, 31 Suncheonhyang 6-gil, Dongnam-gu, Cheonan 31151, Korea E-mail: hbyoung@sch.ac.kr
Copyright © 2020 The Korean Society for Preventive Medicine
This is an Open Access article distributed under the terms of the Creative Commons Attribution Non-Commercial License (http://creativecommons.org/licenses/by-nc/4.0/) which permits unrestricted non-commercial use, distribution, and reproduction in any medium, provided the original work is properly cited.
ABSTRACT
-
Objectives
- This study investigated the effects of comorbid sleep disorders (SD) on the incidence of cardiovascular complications among newly-diagnosed hypertension (HTN) patients.
-
Methods
- As study population, 124 057 newly-diagnosed essential HTN patients aged 30 or older, without cardiovascular complications at diagnosis with HTN, were selected from the National Health Insurance Service-National Sample Cohort. The incidence of cardiovascular complications was calculated, Cox proportional-hazards regression model was used to analyze the risk of complications, and the population attributable fraction (PAF) for cardiovascular complications of having comorbid SD at HTN diagnosis was calculated.
-
Results
- Over 10 years, 32 275 patients (26.0%) developed cardiovascular complications. In HTN patients with comorbid SD at diagnosis of HTN, the incidence of cardiovascular complications (78.3/1000 person-years; 95% confidence interval [CI], 75.8 to 80.9) was higher than in those without comorbid SD (58.6/1000 person-years; 95% CI, 57.9 to 59.3) and the risk of cardiovascular complications was 1.21 times higher (95% CI, 1.17 to 1.25), adjusting for age, gender, income, area of residence, and comorbid diabetes mellitus. The PAF of having comorbid SD at diagnosis of HTN for the incidence of cardiovascular complications was 2.07% (95% CI, 1.69 to 2.44).
-
Conclusions
- Newly-diagnosed essential HTN patients aged 30 or older who had comorbid SD at the time of their HTN diagnosis had a higher incidence of cardiovascular complications than those without comorbid SD. Age, gender, income, area of residence, and comorbid diabetes mellitus had a significant effect on the incidence of cardiovascular complications. Approximately 2% of cardiovascular complications were found to occur due to the presence of SD.
- Keywords: Essential hypertension, Sleep wake disorders, Cardiovascular disease, Incidence
- The number of Korean adults with hypertension (HTN) is estimated to exceed 11 million, which account for 29% of Korean adult population aged 30 or older [1]. The World Health Organization (WHO) has identified raised blood pressure as the leading risk factor for coronary artery disease (CAD), ischemic stroke, and hemorrhagic stroke [2], and inadequately treated HTN as a major risk factor for cardiac disorders and cerebrovascular diseases [3]. As cardiac disorders and cerebrovascular diseases have been the second and third largest causes of death in Korea during 2007-2017 [4], active management and prevention are needed, and HTN, as a causal factor underlying those conditions, also needs appropriate management and prevention.
- Sleep is an important factor that affects the incidence of cardiovascular diseases (CVD) and HTN. Previous studies on the association between sleep duration and HTN and CVD showed that insufficient sleep increased the incidence and mortality of CAD and stroke, at the same time that excessive sleep was a risk factor for the incidence and mortality of CVD, as well as CAD and stroke [5]. In other previous studies, insomnia was found to raise the incidence of heart failure [6] while adequate sleep duration reduced the incidence of obesity, diabetes, HTN, and CVD including stroke [7]. And studies on sleep disorders (SD) such as obstructive sleep apnea [8] and insomnia [9] were found to increase the incidence of CVD and mortality from those conditions, suggesting that inadequate sleep duration and SD affect the incidence of CVD.
- SD, which are defined as a physical and/or psychological nature that cause a sleep disturbance or problem [10], are classified into 7 groups: insomnia, sleep-related breathing disorders, central disorders of hypersomnolence, circadian rhythm sleep-wake disorders, parasomnias, sleep-related movement disorders, and other SD [11]. The International Classification of Diseases, 10th revision (ICD-10) and the Korean Standard Classification of Diseases (KCD) use “G47” and “F51” as the code for SD. In Korea, the number of patients treated for SD has steadily increased, with the number of patients who were treated with the code for SD (G47) reaching 515 326 in 2017 [12]. A cross-sectional study on Korean employees estimated the prevalence of poor quality sleep at 34.2% [13].
- Despite the associations between SD, HTN, and CVD, there are limited data from Korea on the risk of cardiovascular complications in HTN patients with comorbid SD. This study investigated the effects of comorbid SD in newly-diagnosed HTN patients on the incidence of cardiovascular complications through the National Health Insurance Service-National Sample Cohort (NHIS-NSC), with the goal of identifying additional management measures to prevent cardiovascular complications in HTN patients.
INTRODUCTION
- Data and Study Population
- This study used the NHIS-NSC collected between 2002 and 2013. The cohort was established by the National Health Insurance Service to provide public health researchers and policy-makers with representative, useful information regarding Korean citizens’ utilization of health insurance and health examinations. The NHIS-NSC contains data on a randomly extracted sample of 2.2% of the Korean population of 46 605 433 in 2002 [14].
- To analyze diseases, KCD codes were used, with the inclusion of all relevant subcodes. Patients who were diagnosed for HTN (KCD codes: I10, I11, I12, I13, and I15), cardiovascular complications of HTN (hereinafter, referred to as “cardiovascular complications,” KCD codes: I20, I21, I46, I48, I50, I60, I61, I62, I63, and G45), or diseases that could be the cause of cardiovascular complications (KCD codes: N17, N18, N19, I64, I65, I66, I67, I68, and I69) during the 2-year washout period (2002-2003) were excluded from this study. Patients who died during the same period were also excluded from this study. During the next 10 years (2004-2013), patients aged 30 or older who were newly-diagnosed with essential HTN (KCD codes: I10, I11, I12, and I13) were selected, and then patients who had been diagnosed with a condition corresponding to cardiovascular complications before the diagnosis of HTN were excluded. Finally, 124 057 newly-diagnosed essential HTN patients aged 30 or older, who had no cardiovascular complications at the time of diagnosis of HTN, were selected as the study population (Figure 1).
- Variables
- The outcome variable used in this study was the diagnosis of cardiovascular complications (KCD codes: I20, I21, I46, I48, I50, I60, I61, I62, I63, and G45) after being diagnosed with HTN. These codes correspond to angina pectoris (I20), acute myocardial infarction (I21), cardiac arrest (I46), atrial fibrillation and flutter (I48) [15-18], heart failure (I50), subarachnoid hemorrhage (I60), cerebral hemorrhage (I61), other non-traumatic intracranial hemorrhage (I62), cerebral infarction (I63), and transient cerebral ischemia and related syndrome (G45).
- The major independent variable of this study was the presence or absence of SD (KCD codes: F51 and G47) at the time of the HTN diagnosis. As there were no clear grounds for categorizing SD as organic or functional [19], patients were considered as diagnosed for SD in this study if they were diagnosed for either non-organic SD (F51) or SD (G47). The disease codes for SD in the study included the following: non-organic insomnia (F51.0), non-organic hypersomnia (F51.1), non-organic disorder of the sleep-wake schedule (F51.2), sleepwalking [somnambulism] (F51.3), sleep terrors [night terrors] (F51.4), nightmare (F51.5), other non-organic SD (F51.8), non-organic SD, unspecified (F51.9), Disorders of initiating and maintaining sleep [insomnias] (G47.0), disorders of excessive somnolence [hypersomnias] (G47.1), disorders of the sleep-wake schedule (G47.2), sleep apnea (G47.3), narcolepsy and cataplexy (G47.4), other SD (G47.8), and SD, unspecified (G47.9). Patients who were diagnosed with SD and HTN at the same time were classified as having comorbid SD at diagnosis of HTN.
- This study adjusted for the following individual variables and antecedents of patients at diagnosis of HTN: age, gender, income, area of residence, and comorbid diabetes (KCD codes: E10, E11, E12, E13, and E14). The adjustment for comorbid diabetes at the diagnosis of HTN was done as diabetes is one of the major contributing factors for CVD [20,21] and is a common comorbidity of HTN [22]. Patients who were diagnosed with diabetes and HTN at the same time were classified as having comorbid diabetes at diagnosis of HTN. Income levels were divided into low-income (Medical Aid beneficiaries and quantiles 1-4), middle-income (quantiles 5-8), and high-income (quantiles 9-10). Area of residence was divided into large cities (gu-level units of capital of Korea and metropolitan cities), small cities, and rural areas (gun-level unit).
- Statistical Analysis
- Stata/SE version 14.0 (StataCorp., College Station, TX, USA) was used in all statistical analyses with a significance level set to p-value<0.05. The incidence of cardiovascular complications per 1000 person-years was determined by dividing the number of cardiovascular complications cases by the total person-years of the study population with censoring of deaths that occurred during follow-up. The cumulative incidence of cardiovascular complications according to the presence of comorbid SD was analyzed through Kaplan-Meier analysis and the significance of this relationship was verified through the log-rank test. To analyze the effect of SD on cardiovascular complications, hazard ratios (HRs) and 95% confidence intervals (CIs) were calculated using a Cox proportional-hazards model. Three sequential models were analyzed. In model I, age and gender were adjusted. Model II additionally adjusted for income and area of residence. In model III, an additional adjustment for the presence or absence of comorbid diabetes at the diagnosis of HTN was performed on the variables that were used in model II. The population attributable fraction (PAF) and its 95% CI [23] were determined using the Stata command punafcc.
- Ethics Statement
- This study was approved by the Institutional Review Board of the Soonchunhyang University (201712-SB-057-01).
METHODS
Outcome variable
Primary variable of interest
Covariates
- Out of 124 057 newly-diagnosed essential HTN patients aged 30 or older who had no cardiovascular complications when diagnosed with HTN, 32 275 (26.0%) developed cardiovascular complications over the 10-year follow-up period. The general characteristics of the subjects are presented in Table 1.
- The total number of person-years of the study population was 535 344, and the incidence of cardiovascular complications of HTN (hereinafter referred to as the “incidence of complications) per 1000 person-years was 60.3 (95% CI, 59.6 to 61.0) (Table 1). The incidence of complications per 1000 person-years was 29.0 (95% CI, 27.7 to 30.4) for 30- to 39-year-olds, 41.9 (95% CI, 40.9 to 43.0) for 40- to 49-year-olds, 55.8 (95% CI, 54.6 to 57.0) for 50- to 59-year-olds, 78.1 (95% CI, 76.4 to 79.8) for 60- to 69-year-olds, 110.7 (95% CI, 107.8 to 113.6) for 70- to 79-year-olds, and 133.0 (95% CI, 127.3 to 139.1) for those 80 years of age or older, showing that higher age at the diagnosis of HTN was associated with higher incidence of complications. Women (62.2/1000 person-years; 95% CI, 61.3 to 63.2) showed higher incidence of complications than men (58.5/1000 person-years; 95% CI, 57.6 to 59.4). Individuals in the low-income group showed a higher incidence of complications (66.2/1000 person-years; 95% CI, 65.0 to 67.4) than their counterparts in the high-income and middle-income group (60.6/1000 person-years; 95% CI, 59.3 to 61.8 and 55.0/1000 person-years; 95% CI, 54.0 to 56.0, respectively). The incidence of complications was highest in rural areas (77.0/1000 person-years; 95% CI, 74.9 to 79.2), followed by small cities (59.9/1000 person-years; 95% CI, 58.9 to 60.9), and large cities showed the lowest rate (56.2/1000 person-years; 95% CI, 55.2 to 57.1). Patients with comorbid SD at the diagnosis of HTN (78.3/1000 person-years; 95% CI, 75.8 to 80.9) showed a higher incidence of complications than those without comorbid SDs (58.6/1000 person-years; 95% CI, 57.9 to 59.3). Patients with comorbid diabetes at the diagnosis of HTN (73.7/1000 person-years; 95% CI, 72.2 to 75.3) also showed higher incidence of complications than those who did not have comorbid diabetes (56.3/1000 person-years; 95% CI, 55.6 to 57.1).
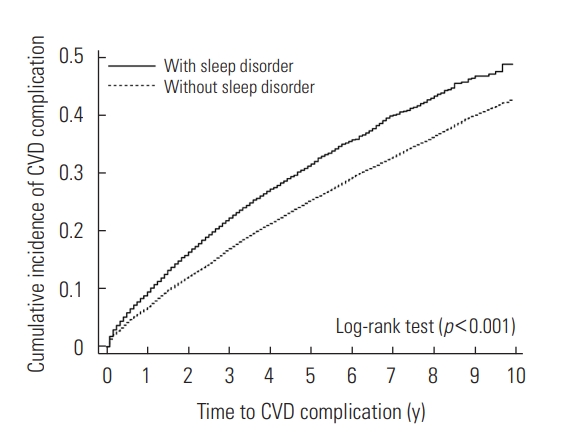
- The cumulative incidence of complications according to the presence of comorbid SD at the diagnosis of HTN is presented in Figure 2 (log-rank test, p<0.001).
- When adjusting for age and gender at the diagnosis of HTN, HTN patients with comorbid SD had a higher risk of cardiovascular complications (1.23; 95% CI, 1.19 to 1.27) than those without comorbid SD (model 1). When income and area of residence were additionally corrected, HTN patients with comorbid SD continued to have a higher risk of cardiovascular complications (1.23; 95% CI, 1.19 to 1.27; model 2). With additional adjustment for diabetes, HTN patients with comorbid SD still had a higher risk of cardiovascular complications (1.21; 95% CI, 1.17 to 1.25) than their counterparts (Table 2).
- The PAF for the incidence of cardiovascular complications of having comorbid SD at the diagnosis of HTN was 2.07% (95% CI, 1.69 to 2.44). Among other major risk factors in this study, PAF was highest for age of 65 or older (17.79%; 95% CI, 17.16 to 18.42), followed by comorbid diabetes at the diagnosis of HTN, residence in a small city or rural area, and low income, with PAFs of 5.31% (95% CI, 4.68 to 5.93), 4.16% (95% CI, 2.92 to 5.38), and 3.26% (95% CI, 2.45 to 4.05), respectively. Gender had lower PAF than SD which accounted for 1.90% (95% CI, 0.82 to 2.97). The results of this study showed that approximately 2.07% of cardiovascular complications that occurred in patients with newly-diagnosed essential HTN, aged 30 or older, and without cardiovascular complications at the diagnosis of HTN were attributable to SD (p<0.001) (Table 3).
RESULTS
- The results of this study show that patients who had comorbid SD at the diagnosis of HTN had a significantly higher risk of cardiovascular complications than those without comorbid SD. Despite a comprehensive literature search, no studies were found that analyzed the impact of comorbid SD on the incidence of cardiovascular complications in HTN patients, as was done in this study. Since the codes for SD used in this study included various SD, such as insomnia (F51.0, G47.0), hypersomnia (F51.1, G47.1), sleep apnea (G47.3), and nightmares (F51.5), and cardiovascular complications included various heart and CVD, the results of this study are in line with those of previous studies reporting that inappropriate sleep hours increased the occurrence of CAD or stroke and resultant mortality [5], that obstructive sleep apnea [8,24] and insomnia [9] increased cardiovascular complications and mortality, and that nightmares increased the risk of myocardial infarctions occurring during sleep [25]. In addition, literature reviews of SD [26-28] has suggested that primary sleep abnormalities such as curtailed sleep, shift work, and sleep-disordered breathing might have a causal association with atherosclerosis, stroke, heart failure, and cardiac arrhythmia [26]. It has also been reported that inadequate sleep duration and SD such as obstructive sleep apnea and sleep-related movement disorder might be risk factors of stroke [27]. SD were also pervasive in stroke patients and the patients at risk for stroke [28]. The results of this study are compatible to the findings of previous studies.
- In this study, the PAF of having comorbid SD at the diagnosis of HTN for the incidence of cardiovascular complications was 2.07%. This suggests that approximately 1 out of 50 cases of cardiovascular complications that occurred in HTN patients was caused by SD. Therefore, if comorbid SD are properly treated in HTN patients, cardiovascular risk could be decreased. These results are compatible with previous studies that suggest the treatment of SD may improve further stroke risk [29].
- This study has the following limitations. First, as the concordance rate between 3-digit disease codes in Korean National Health Insurance claims and actual medical records is 82.0% for main diagnoses, and that of 4-digit or 5-digit disease codes is known to be lower [30], only using KCD codes for establishing diagnoses poses a risk of misclassification bias. Second, the exposure in the study was set as a disease group (SD) rather than specific disease entities. So some specific SD that might not have a clear association with cardiovascular complications may have been included in the disease group. Nonetheless, the resultant possibility of the results being distorted is unlikely, as these factors would skew the findings toward the null hypothesis. Third, since the NHIS-NSC is based on insurance claims, control of disease was not part of the scope of the database thus adjustment for control of disease was not possible.
- Despite these limitations, this study has its strengths. First, the study conducted population-based research [14] using a nationally representative sample, and observed the incidence of cardiovascular complications for a long period, up to 10 years, after the diagnosis of HTN. Second, this is the first study to analyze the effects of comorbid SD on the incidence of cardiovascular complications among newly-diagnosed Korean HTN patients. Third, the results of this study are based on newly-diagnosed, uncomplicated HTN patients at the point of diagnosis. In consideration of low medication adherence among Korean hypertensives [31], particularly in newly-diagnosed HTN patients without prior complications [32], the results of this study can be used to improve management and treatment of HTN and SD, especially for newly-diagnosed patients, to promote better adherence to treatment, leading to better health outcomes.
- In this study, HTN patients with comorbid SD at the time of their HTN diagnosis had a significantly higher incidence of cardiovascular complications than their counterparts, even after adjusting for age, gender, income, area of residence, and comorbid diabetes at the time of the HTN diagnosis. The result of study shows that appropriate treatment of SD among HTN patients could reduce cardiovascular risk. These results imply that the presence of SD should be considered as a risk factor for cardiovascular complications among newly-diagnosed HTN patients. Comorbid SD in newly-diagnosed HTN patients should receive attention in terms of treatment, education, and prevention of complications. Furthermore, evaluating SD through instruments such as the Pittsburgh Sleep Quality Index, Epworth Sleepiness Scale, and the Berlin questionnaire at the diagnosis of HTN and appropriate treatment of SD will be helpful for reducing the high burden of cardiovascular complications that result from HTN.
DISCUSSION
-
CONFLICT OF INTEREST
The authors have no conflicts of interest associated with the material presented in this paper.
-
FUNDING
None.
Notes
ACKNOWLEDGEMENTS
-
AUTHOR CONTRIBUTIONS
Conceptualization: JK, YH. Data curation: JK, YHP, YH. Formal analysis: JK, YH. Funding acquisition: None. Methodology: JK, YH. Project administration: YHP, YH. Visualization: JK. Writing - original draft: JK. Writing - review & editing: JK, YHP, KIY, JRBC, YH.
Notes

| Characteristics | Study population (n=124 057) |
Incident CVD, n (%) |
Person-years | CVD incidence per 1000 person-year (95% CI) | |
|---|---|---|---|---|---|
| No (n=91 782) | Yes (n=32 275) | ||||
| Age (y) | |||||
| 30-39 | 13 504 | 11 634 (86.2) | 1870 (13.9) | 64 479 | 29.0 (27.7, 30.4) |
| 40-49 | 32 180 | 25 946 (80.6) | 6234 (19.4) | 148 637 | 41.9 (40.9, 43.0) |
| 50-59 | 35 592 | 27 086 (76.1) | 8506 (23.9) | 152 563 | 55.8 (54.6, 57.0) |
| 60-69 | 24 636 | 16 402 (66.6) | 8234 (33.4) | 105 470 | 78.1 (76.4, 79.8) |
| 70-79 | 13 409 | 7918 (59.1) | 5491 (41.0) | 49 613 | 110.7 (107.8, 113.6) |
| ≥80 | 4736 | 2796 (59.0) | 1940 (41.0) | 14 582 | 133.0 (127.3, 139.1) |
| Gender | |||||
| Men | 64 382 | 48 339 (75.1) | 16 043 (24.9) | 274 396 | 58.5 (57.6, 59.4) |
| Women | 59 675 | 43 443 (72.8) | 16 232 (27.2) | 260 948 | 62.2 (61.3, 63.2) |
| Income | |||||
| Low | 43 653 | 31 716 (72.7) | 11 937 (27.4) | 180 315 | 66.2 (65.0, 67.4) |
| Middle | 47 180 | 35 682 (75.6) | 11 498 (24.4) | 209 034 | 55.0 (54.0, 56.0) |
| High | 33 224 | 24 384 (73.4) | 8840 (26.6) | 145 995 | 60.6 (59.3, 61.8) |
| Area of residence | |||||
| Large city | 54 186 | 40 764 (75.2) | 13 422 (24.8) | 238 925 | 56.2 (55.2, 57.1) |
| Small city | 54 309 | 40 432 (74.5) | 13 877 (25.6) | 231 833 | 59.9 (58.9, 60.9) |
| Rural | 15 562 | 10 586 (68.0) | 4976 (32.0) | 64 586 | 77.0 (74.9, 79.2) |
| Comorbidity | |||||
| SD | |||||
| No | 110 011 | 81 352 (74.0) | 28 659 (26.1) | 489 187 | 58.6 (57.9, 59.3) |
| Yes | 14 046 | 10 430 (74.3) | 3616 (25.7) | 46 157 | 78.3 (75.8, 80.9) |
| SD (G47)1 | 6656 | 5129 (77.1) | 1527 (22.9) | 21 055 | 72.5 (69.0, 76.3) |
| NSD (F51)2 | 2712 | 2185 (80.6) | 527 (19.4) | 8856 | 59.5 (54.6, 64.8) |
| Both3 | 4678 | 3116 (66.6) | 1562 (33.4) | 16 247 | 96.1 (91.5, 101.0) |
| Diabetes mellitus | |||||
| No | 92 526 | 69 218 (74.8) | 23 308 (25.2) | 413 684 | 56.3 (55.6, 57.1) |
| Yes | 31 531 | 22 564 (71.6) | 8967 (28.4) | 121 659 | 73.7 (72.2, 75.3) |
| Total | 124 057 | 91 782 (74.0) | 32 275 (26.0) | 535 344 | 60.3 (59.6, 61.0) |
HTN, hypertension; CVD, cardiovascular diseases; CI, confidence interval; SD, sleep disorders; NSD, non-organic sleep disorders; KCD, Korean Standard Classification of Diseases.
1 Patients with only the KCD code G47 (SD) or subcodes at the diagnosis of HTN.
2 Patients with only the KCD code F51 (NSD) or subcodes at the diagnosis of HTN.
3 Patients with both the KCD codes G47 and F51 or subcodes at the diagnosis of HTN.
- 1. Korean Society Hypertension. Korea hypertension fact sheet 2018 [cited 2019 Sep 1]. Available from: http://www.koreanhypertension.org/reference/guide?mode=read&idno=4166 (Korean)
- 2. World Health Organization. Raised blood pressure [cited 2019 Sep 1]. Available from: https://www.who.int/gho/ncd/risk_factors/blood_pressure_prevalence_text/en/
- 3. Harrison TR, Isselbacher KJ, Wilson JD. Harrison’s principles of internal medicine. 19th ed. New York: McGraw-Hill; 2015. p. 1825
- 4. Statistics Korea. Cause of death in Korea 2017 [cited 2019 Aug 14]. Available from: http://kostat.go.kr/portal/korea/kor_nw/1/6/2/index.board?bmode=read&aSeq=370710&pageNo=1&rowNum=10&amSeq=&sTarget=&sTxt= (Korean)
- 5. Cappuccio FP, Cooper D, D’Elia L, Strazzullo P, Miller MA. Sleep duration predicts cardiovascular outcomes: a systematic review and meta-analysis of prospective studies. Eur Heart J 2011;32(12):1484-1492ArticlePubMedPDF
- 6. Laugsand LE, Strand LB, Platou C, Vatten LJ, Janszky I. Insomnia and the risk of incident heart failure: a population study. Eur Heart J 2014;35(21):1382-1393ArticlePubMedPDF
- 7. Buxton OM, Marcelli E. Short and long sleep are positively associated with obesity, diabetes, hypertension, and cardiovascular disease among adults in the United States. Soc Sci Med 2010;71(5):1027-1036ArticlePubMed
- 8. Marin JM, Carrizo SJ, Vicente E, Agusti AG. Long-term cardiovascular outcomes in men with obstructive sleep apnoea-hypopnoea with or without treatment with continuous positive airway pressure: an observational study. Lancet 2005;365(9464):1046-1053ArticlePubMed
- 9. Sofi F, Cesari F, Casini A, Macchi C, Abbate R, Gensini GF. Insomnia and risk of cardiovascular disease: a meta-analysis. Eur J Prev Cardiol 2014;21(1):57-64ArticlePubMed
- 10. Stores G, Wiggs L. Sleep disturbance in children and adolescents with disorders of development: its significance and management. London: Cambridge University Press; 2001. p. 16
- 11. American Academy of Sleep Medicine. International classification of sleep disorders. 2014 [cited 2019 Sep 1]. Available from: https://j2vjt3dnbra3ps7ll1clb4q2-wpengine.netdna-ssl.com/wp-content/uploads/2019/05/ICSD3-TOC.pdf
- 12. HIRA Web Magazine. Theme disease – sleep disorder. 2018 Jun 15 [cited 2019 Aug 14]. Available from: https://m.post.naver.com/viewer/postView.nhn?volumeNo=16050604&memberNo=1891127&searchKeyword=%EC%83%9D%EC%B2%B4%EC%8B%9C%EA%B3%84&searchRank=340 (Korean)
- 13. Woo JM, Hyun SY, Lee SH, Kang SG, Lee JS, Kim L, et al. Productivity time lost by sleep disturbance among workers in Korea. J Korean Neuropsychiatr Assoc 2011;50(1):62-68. (Korean)
- 14. Lee J, Lee JS, Park SH, Shin SA, Kim K. Cohort profile: the National Health Insurance Service-National Sample Cohort (NHIS-NSC), South Korea. Int J Epidemiol 2017;46(2):e15ArticlePubMedPDF
- 15. Kannel WB, Wolf PA, Benjamin EJ, Levy D. Prevalence, incidence, prognosis, and predisposing conditions for atrial fibrillation: population-based estimates. Am J Cardiol 1998;82(8A):2N-9NArticlePubMed
- 16. Allan V, Honarbakhsh S, Casas JP, Wallace J, Hunter R, Schilling R, et al. Are cardiovascular risk factors also associated with the incidence of atrial fibrillation? A systematic review and field synopsis of 23 factors in 32 population-based cohorts of 20 million participants. Thromb Haemost 2017;117(5):837-850ArticlePubMedPMCPDF
- 17. Dzeshka MS, Shantsila A, Shantsila E, Lip GY. Atrial fibrillation and hypertension. Hypertension 2017;70(5):854-861ArticlePubMed
- 18. Saksena S, Camm AJ. Electrophysiological disorders of the heart. 2nd ed. Philadelphia: Elsevier Health Sciences; 2011. p. 572
- 19. White PD, Rickards H, Zeman AZ. Time to end the distinction between mental and neurological illnesses. BMJ 2012;344: e3454ArticlePubMed
- 20. Grundy SM, Benjamin IJ, Burke GL, Chait A, Eckel RH, Howard BV, et al. Diabetes and cardiovascular disease: a statement for healthcare professionals from the American Heart Association. Circulation 1999;100(10):1134-1146ArticlePubMed
- 21. Leon BM, Maddox TM. Diabetes and cardiovascular disease: epidemiology, biological mechanisms, treatment recommendations and future research. World J Diabetes 2015;6(13):1246-1258ArticlePubMedPMC
- 22. Sowers JR, Epstein M, Frohlich ED. Diabetes, hypertension, and cardiovascular disease: an update. Hypertension 2001;37(4):1053-1059ArticlePubMed
- 23. Newson RB. Attributable and unattributable risks and fractions and other scenario comparisons. Stata J 2013;13(4):672-698Article
- 24. Dyken ME, Im KB. Obstructive sleep apnea and stroke. Chest 2009;136(6):1668-1677ArticlePubMed
- 25. Selvi Y, Aydin A, Gumrukcuoglu HA, Gulec M, Besiroglu L, Ozdemir PG, et al. Dream anxiety is an emotional trigger for acute myocardial infarction. Psychosomatics 2011;52(6):544-549ArticlePubMed
- 26. Wolk R, Gami AS, Garcia-Touchard A, Somers VK. Sleep and cardiovascular disease. Curr Probl Cardiol 2005;30(12):625-662ArticlePubMed
- 27. Koo DL, Nam H, Thomas RJ, Yun CH. Sleep disturbances as a risk factor for stroke. J Stroke 2018;20(1):12-32ArticlePubMedPMCPDF
- 28. Wallace DM, Ramos AR, Rundek T. Sleep disorders and stroke. Int J Stroke 2012;7(3):231-242ArticlePubMedPMC
- 29. Mims KN, Kirsch D. Sleep and stroke. Sleep Med Clin 2016;11(1):39-51ArticlePubMed
- 30. Park E. Assessment on concictency of KCD codes and medical record and methods for improvement. Wonju: Health Insurance Review and Assessment Service; 2017. p. 79-81 (Korean)
- 31. Ah YM, Lee JY, Choi YJ, Kim B, Choi KH, Kong J, et al. Persistence with antihypertensive medications in uncomplicated treatment-naïve patients: effects of initial therapeutic classes. J Korean Med Sci 2015;30(12):1800-1806ArticlePubMedPMC
- 32. Lee SG, Jeon SY. The knowledge, attitude and practice of blood pressure management from the patient’s viewpoint: a qualitative study. J Prev Med Public Health 2008;41(4):255-264. (Korean)ArticlePubMedPDF
REFERENCES
Figure & Data
References
Citations

- Association Between Sleep Quality and Anxiety in Korean Adolescents
Hyunkyu Kim, Seung Hoon Kim, Sung-In Jang, Eun-Cheol Park
Journal of Preventive Medicine and Public Health.2022; 55(2): 173. CrossRef
 KSPM
KSPM

 PubReader
PubReader ePub Link
ePub Link Cite
Cite



