Articles
- Page Path
- HOME > J Prev Med Public Health > Volume 53(1); 2020 > Article
-
Original Article
Association Between Atrial Fibrillation and the Risk of Dementia in the Korean Elderly: A 10-Year Nationwide Cohort Study -
Min-Ah Nah
 , Kyeong Soo Lee
, Kyeong Soo Lee , Tae-Yoon Hwang
, Tae-Yoon Hwang
-
Journal of Preventive Medicine and Public Health 2020;53(1):56-63.
DOI: https://doi.org/10.3961/jpmph.19.117
Published online: January 3, 2020
Department of Preventive Medicine and Public Health, Yeungnam University College of Medicine, Daegu, Korea
- Corresponding author: Kyeong Soo Lee, MD, PhD Department of Preventive Medicine and Public Health, Yeungnam University College of Medicine, 170 Hyeonchung-ro, Nam-gu, Daegu 42415, Korea E-mail: drkslee@ynu.ac.kr
Copyright © 2020 The Korean Society for Preventive Medicine
This is an Open Access article distributed under the terms of the Creative Commons Attribution Non-Commercial License (http://creativecommons.org/licenses/by-nc/4.0/) which permits unrestricted non-commercial use, distribution, and reproduction in any medium, provided the original work is properly cited.
ABSTRACT
-
Objectives
- The objective of this study was to determine the effect of atrial fibrillation (AF) on the risk of dementia in the Korean elderly.
-
Methods
- A 10-year retrospective cohort study was conducted using the National Health Insurance Service-Senior Cohort database. We excluded those who were under 65 years of age as of January 2006 (n=46 113), those who were diagnosed with dementia between 2002 and 2005 (n=9086), and those with a history of stroke prior to AF diagnosis (n=8392). We used a Cox proportional hazards model with a time-varying covariate to determine whether AF is associated with the risk of dementia after adjusting for potential confounders.
-
Results
- In univariable Cox regression, the hazard ratio (HR) of dementia according to AF status was 1.28 (95% confidence interval [CI], 1.23 to 1.33). After adjusting for potential confounders, AF was found to increase the risk of dementia (HR, 1.12; 95% CI, 1.07 to 1.17), Alzheimer dementia (HR, 1.12; 95% CI, 1.07 to 1.17), and vascular dementia (HR, 1.10; 95% CI, 1.03 to 1.18). In patients diagnosed with AF, the incidence of dementia was lower (HR, 0.50; 95% CI, 0.47 to 0.52) in patients who were treated with oral anticoagulants.
-
Conclusions
- Investigating the potential risk factors of dementia in an aged society is important. We found a slightly higher risk of dementia in those with AF than in those without AF, and we therefore concluded that AF is a potential risk factor for dementia.
- Korea became an aged society in 2017, with elderly citizens (aged 65 and up) accounting for over 14% of the population. The prevalence of dementia, a disorder predominantly affecting elderly individuals, is also on the rise as the population ages. More than 10% of those aged 65 and over had dementia in 2017, and the number of patients with dementia is expected to surpass 1 million nationwide by 2024 [1]. The level of socioeconomic burden posed by the disease also continues to increase, with the cost of managing dementia projected to reach 18.8 trillion Korean won by 2020 [1]. Against this backdrop, it is important to prioritize the development of primary preventive policies in addition to ways to manage existing patients; in the service of this goal, further studies are necessary to clarify the potential risk factors for dementia.
- Risk factors for dementia include socio-demographic factors, such as age and sex; genetic factors; physical and mental health factors, such as hypertension, diabetes, and depression; and lifestyle habits, such as smoking and drinking [2-4]. Many studies of community-based cohorts in Europe and the United States have found that atrial fibrillation (AF) is an independent risk factor for dementia [5-11], while some studies have reported that AF increases not only the risk of vascular dementia, but also that of Alzheimer dementia.
- AF is the most common type of chronic arrhythmia and shares risk factors with dementia, including age, hypertension, diabetes, and smoking. Additionally, AF is a risk factor for ischemic stroke, which is known to cause vascular dementia [12]. Studies are being conducted to understand the mechanisms by which AF independently causes dementia [13], with hypotheses including asymptomatic cerebral infarction [14], chronic cerebral hypoperfusion [15], inflammation [16,17], decreased brain volume [18], and microbleeds due to anticoagulant use. Meanwhile, other studies have claimed that treatment with catheter ablation [19] as well as the use of oral coagulants in AF patients can actually reduce the risk of dementia [20]. Further studies are needed to assess the association between the use of coagulants and the occurrence of dementia.
- Outside of Korea, many studies have already probed the association between AF and dementia. However, this has not been the case in Asian countries, including Korea, despite the fact that the epidemiological characteristics of AF and dementia varies by country and ethnic group [21-25]. AF and dementia are chronic diseases that are affected by lifestyle habits [26-28] and genetic factors [29-31], so they are affected by racial and cultural factors. Therefore, it is crucial to study this topic in the Korean population, rather than simply applying the results of studies conducted abroad.
- For this study, we used the National Health Insurance Service-Senior Cohort (NHIS-SC) database, which accurately represents the nation’s population and has a large sample size, to assess whether AF acts as an independent risk factor for dementia, Alzheimer dementia, and vascular dementia. And we also examined the impact of anticoagulant use on the dementia risk of AF patients.
INTRODUCTION
- Data Source and Study Population
- This study analyzed data from the NHIS-SC. The NHIS-SC database consists of 14 years (2002-2015) of cumulative records of 550 000 randomly-selected members of the senior population aged 60 years and older more regarding their use of medical services. This sample data accounts for approximately 10% of the 5 581 470-member senior population of Korea. The personal information of the individuals was not identifiable.
- The study determined the washout period of dementia to be 4 years; therefore, of the 14 years of follow-up data, we analyzed dementia risk over the 10-year period from 2006 to 2015. From the total cohort of 558 147 seniors, those who were not eligible for National Health Insurance or Medical Aid as of January 2006 (n=53 730) for reasons such as death and those under 65 years of age (n=46 113) were excluded. A final sample of 440 826 subjects were included in the analysis after excluding subjects who had been diagnosed with dementia between 2002 and 2005 (n=9086) and subjects with a history of stroke prior to diagnosis of AF (n=8392) (Figure 1).
- Operational Definitions of Diseases
- Cases of dementia, AF, and other comorbidities were defined as cases with the disease in question listed as the main diagnosis or sub-diagnosis based on the Korean Standard Classification of Diseases. The code for AF (the main independent variable) was I48, the codes for dementia were F00-F03, and the codes for Alzheimer and vascular dementia were F00 and F01, respectively. The codes for hypertension were I10-I15, the codes for diabetes were E10-E14, those for stroke were I60-I63, those for post-stroke symptoms were I690-I694, the code for heart failure was I50, the code for ischemic heart disease was I24, and the codes for valvular heart diseases were I05-I08 and I34-I37. Stroke was limited to cases for which the insurance claim was classified as inpatient and magnetic resonance imaging or computed tomography examination was performed.
- Anticoagulant use was defined as the prescription of drugs that contained the main ingredient codes for wafarin and direct oral anticoagulants. The main ingredient codes were 249101ATB, 249102ATB, 249103ATB, 249104ATB, 249105ATB, 249106ATB, 249107ATB, 249108ATB, 249109ATB, 511401ATB, 511402ATB, 511403ATB, 511404ATB, 613701ACH, 613702ACH, 613703ACH, 617001ATB, 617002ATB, 643601ATB, 643602ATB, and 643603ATB.
- Statistical Analysis
- The general characteristics of the subjects were identified using the t-test and the chi-square test. A Cox proportional hazards model was used with the independent variable of AF status as a time-varying covariate over the 10-year follow-up study period to explore the association between AF and dementia. The adjusted confounders were age, sex, hypertension, diabetes, heart failure, ischemic heart disease, and valvular heart disease. The same method was used to assess whether AF acted as an independent risk factor for Alzheimer and vascular dementia, as well as whether anticoagulant use affected the development of dementia among AF patients.
- All statistical analyses were performed with SAS version 9.4 (SAS Institute Inc., Cary, NC, USA).
- Ethics Statement
- This study was approved by the Institutional Review Board of Yeungnam University Hospital (YUMC-2018-08-009).
METHODS
- Of the total of 440 826 subjects, 4631 (1.1%) patients had already been diagnosed with AF as of January 2006, a total of 29 831 (6.8%) patients were newly diagnosed with AF between 2006 and 2015, and 406 364 (92.2%) subjects were not diagnosed with AF (Table 1).
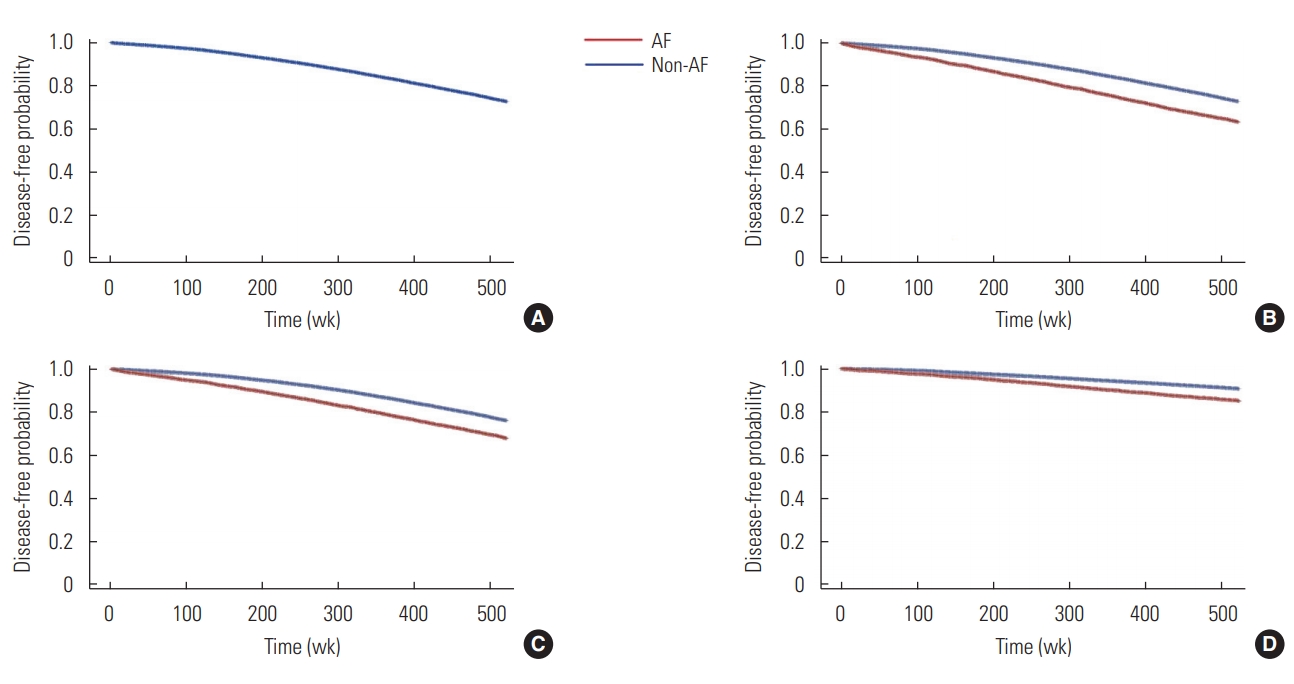
- The Cox regression hazard model with AF status as a time-varying covariate showed that age, sex, hypertension, diabetes, heart failure, ischemic heart disease, and valvular heart disease were each meaningfully associated with the risk of dementia when not adjusted for the effects across the variables. The hazard ratio (HR) of dementia according to AF status was 1.28 (95% confidence interval [CI], 1.23 to 1.33). The multivariable analysis, which adjusted for age, sex, hypertension, diabetes, heart failure, ischemic heart disease, and valvular heart disease, showed that having AF slightly increased the risk of dementia (HR, 1.12; 95% CI, 1.07 to 1.17) (Table 2, Figure 2A and 2B). When we adjusted for the same variables to assess the associations between AF and Alzheimer dementia and between AF and vascular dementia, AF was found to be significantly associated with both Alzheimer dementia (HR, 1.12; 95% CI, 1.07 to 1.17) and vascular dementia (HR, 1.10; 95% CI, 1.03 to 1.18) (Table 2, Figure 2C and 2D).
- Among AF patients, those who used oral anticoagulants had a statistically significantly lower risk of developing dementia (HR, 0.50; 95% CI, 0.47 to 0.52). Anticoagulant use was also confirmed to reduce the risk of Alzheimer dementia (HR, 0.48; 95% CI, 0.45 to 0.51) and vascular dementia (HR, 0.55; 95% CI, 0.50 to 0.60) (Table 3).
RESULTS
- This study is novel for its attempt to epidemiologically understand the potential risk factors for dementia by analyzing the association between AF and dementia. The use of National Health Insurance Service (NHIS) sample cohort data improved the sample size and representation of the subjects, while also ensuring a sufficient follow-up period. Since the NHIS sample cohort data are secondary data collected for the purpose of administrative management, it was important to clarify the standards used to define diseases and morbidity, as well as the selection method of subjects. In this study, the washout period was set at 4 years, given that the 99th percentile of the average time to diagnosis in patients diagnosed with dementia was 38 months, to rule out subjects who had already been diagnosed with dementia. Subjects who had already been diagnosed with stroke before the diagnosis of AF were also excluded to eliminate cases of dementia caused by stroke preceding AF.
- This study verified that the association between AF and dementia was statistically significant when controlling for sex, age, hypertension, diabetes, heart failure, and valvular heart disease. The association between AF and dementia has 2 explanations. First, AF and dementia share common risk factors, such as sex, age, lifestyle habits, hypertension, diabetes, and heart disease [3,4,32-34], and second, AF increases the risk of dementia through a number of potential mechanisms. The results of this study serve as the foundation for the second explanation, that AF itself may contribute to an increased risk of dementia. The results also showed that the development of dementia, Alzheimer dementia, and vascular dementia was meaningfully associated with AF status even when controlling for sex, age, and comorbidities. This aligns with preceding meta-analyses [35-37] as well as registry-based studies [6,8,37]. These results can be complemented by research on potential mechanisms by which AF may increase the risk of dementia. Suggested mechanisms include cerebral hypoperfusion due to low cardiac output [15], asymptomatic stroke due to a small thrombus [14], and activation of inflammation [17].
- Many preceding studies [38,39] have found that the use of anticoagulants lowers the risk of deterioration of cognitive functions. The suspected mechanism behind this is that the use of anticoagulants can prevent blood clots that can lead to symptomatic and asymptomatic stroke [39]. This study also confirmed that the use of anticoagulants lowered the risk of developing dementia, Alzheimer’s dementia, and vascular dementia. From this, it is safe to assume that the consistent use of anticoagulants in AF patients may help lower the risk of developing dementia and the associated deterioration of cognitive functions.
- The subjects of this study were senior Korean citizens aged 65 and older. Given that AF and dementia are becoming increasingly prevalent among the middle-aged [21,40] and that a previous study has shown a higher risk of developing dementia among younger AF patients [5], future studies should be carried out on a broader population base that includes younger population to verify whether AF raises the risk of dementia among these subjects as well.
- Meanwhile, unlike previous studies [5,8,12,37] such as the Framingham Heart Study, which used health examination data collected for the purpose of the study, the NHIS elderly sample cohort database was originally collected for the purposed of reviewing medical claims under disease diagnosis codes. This implies limitations in accurately assessing morbidity and prevalence based on symptoms, signs, and diagnostic exam results. Surveys and diagnosis-based research should be carried out in the future to ensure a more detailed define for target diseases.
- Despite the above limitations, this study attempted to move towards obtaining an epidemiological explanation of dementia among AF patients by utilizing access to the big sample data regarding the use of medical services available from the NHIS senior citizen cohort database. The results of this study confirmed that AF can potentially increase the risk of dementia, Alzheimer dementia, and vascular dementia, and that the intake of oral anticoagulants lowers the risk of developing dementia among AF patients in Korea.
DISCUSSION
-
CONFLICT OF INTEREST
The authors have no conflicts of interest associated with the material presented in the paper.
-
FUNDING
None.
Notes
ACKNOWLEDGEMENTS
-
AUTHOR CONTRIBUTIONS
Conceptualization: MAN, KSL, TYH. Data curation: MAN. Formal analysis: MAN. Funding acquisition: None. Methodology: MAN, KSL. Project administration: KSL. Visualization: MAN. Writing-original draft: MAN, KSL, TYH. Writing - review & editing: MAN, KSL, TYH.
Notes


| Variables |
Dementia |
Alzheimer dementia |
Vascular dementia |
|||||
|---|---|---|---|---|---|---|---|---|
| Univariable Cox (unadjusted) | p-value | Multivariable Cox (adjusted)1 | p-value | Multivariable Cox (adjusted)1 | p-value | Multivariable Cox (adjusted)1 | p-value | |
| Age | 1.08 (1.08, 1.09) | <0.001 | 1.08 (1.08, 1.08) | <0.001 | 1.09 (1.09, 1.09) | <0.001 | 1.08 (1.08, 1.08) | <0.001 |
| Sex | ||||||||
| Male | 1.00 (reference) | 1.00 (reference) | 1.00 (reference) | 1.00 (reference) | ||||
| Female | 1.47 (1.45, 1.49) | <0.001 | 1.26 (1.25, 1.28) | <0.001 | 1.31 (1.29, 1.32) | <0.001 | 1.08 (1.06, 1.11) | <0.001 |
| Hypertension | ||||||||
| No | 1.00 (reference) | 1.00 (reference) | 1.00 (reference) | 1.00 (reference) | ||||
| Yes | 1.33 (1.31, 1.34) | <0.001 | 1.14 (1.13, 1.16) | <0.001 | 1.10 (1.08, 1.12) | <0.001 | 1.36 (1.33, 1.40) | <0.001 |
| Diabetes mellitus | ||||||||
| No | 1.00 (reference) | 1.00 (reference) | 1.00 (reference) | 1.00 (reference) | ||||
| Yes | 1.23 (1.21, 1.24) | <0.001 | 1.23 (1.21, 1.25) | <0.001 | 1.23 (1.21, 1.25) | <0.001 | 1.28 (1.25, 1.31) | <0.001 |
| Heart failure | ||||||||
| No | 1.00 (reference) | 1.00 (reference) | 1.00 (reference) | 1.00 (reference) | ||||
| Yes | 1.39 (1.36, 1.41) | <0.001 | 1.08 (1.06, 1.10) | <0.001 | 1.09 (1.07, 1.11) | <0.001 | 1.09 (1.05, 1.12) | <0.001 |
| Ischemic heart disease | ||||||||
| No | 1.00 (reference) | 1.00 (reference) | 1.00 (reference) | 1.00 (reference) | ||||
| Yes | 1.20 (1.18, 1.22) | <0.001 | 1.05 (1.04, 1.07) | <0.001 | 1.05 (1.03, 1.07) | <0.001 | 1.07 (1.04, 1.10) | <0.001 |
| Valvular heart disease | ||||||||
| No | 1.00 (reference) | 1.00 (reference) | 1.00 (reference) | 1.00 (reference) | ||||
| Yes | 1.31 (1.20, 1.43) | <0.001 | 1.02 (0.93, 1.12) | 0.697 | 1.02 (0.92, 1.13) | 0.697 | 1.10 (0.94, 1.28) | 0.215 |
| Atrial fibrillation (time-varying covariate) | ||||||||
| No | 1.00 (reference) | 1.00 (reference) | 1.00 (reference) | 1.00 (reference) | ||||
| Yes | 1.28 (1.23, 1.33) | <0.001 | 1.12 (1.07, 1.17) | <0.001 | 1.12 (1.07, 1.17) | <0.001 | 1.10 (1.03, 1.18) | 0.004 |
| Diseases | HR (95% CI)1 | p-value |
|---|---|---|
| Dementia | 0.50 (0.47, 0.52) | <0.001 |
| Alzheimer dementia | 0.48 (0.45, 0.51) | <0.001 |
| Vascular dementia | 0.55 (0.50, 0.60) | <0.001 |
- 1. Ministry of Health and Welfare. Korean dementia observatory 2018 [cited 2019 Feb 14]. Available from: https://www.nid.or.kr/info/dataroom_view.aspx?bid=194 (Korean)
- 2. Skoog I. Status of risk factors for vascular dementia. Neuroepidemiology 1998;17(1):2-9ArticlePubMed
- 3. Livingston G, Sommerlad A, Orgeta V, Costafreda SG, Huntley J, Ames D, et al. Dementia prevention, intervention, and care. Lancet 2017;390(10113):2673-2734ArticlePubMed
- 4. Scheltens P, Blennow K, Breteler MM, de Strooper B, Frisoni GB, Salloway S, et al. Alzheimer’s disease. Lancet 2016;388(10043):505-517ArticlePubMed
- 5. de Bruijn RF, Heeringa J, Wolters FJ, Franco OH, Stricker BH, Hofman A, et al. Association between atrial fibrillation and dementia in the general population. JAMA Neurol 2015;72(11):1288-1294ArticlePubMed
- 6. Bunch TJ, Weiss JP, Crandall BG, May HT, Bair TL, Osborn JS, et al. Atrial fibrillation is independently associated with senile, vascular, and Alzheimer’s dementia. Heart Rhythm 2010;7(4):433-437ArticlePubMed
- 7. Rusanen M, Kivipelto M, Levälahti E, Laatikainen T, Tuomilehto J, Soininen H, et al. Heart diseases and long-term risk of dementia and Alzheimer’s disease: a population-based CAIDE study. J Alzheimers Dis 2014;42(1):183-191ArticlePubMed
- 8. Rudarakanchana N, Hamady M, Harris S, Afify E, Gibbs R, Bicknell CD, et al. Early outcomes of patients transferred with ruptured suprarenal aneurysm or dissection. Ann R Coll Surg Engl 2018;100(4):316-321ArticlePubMedPMC
- 9. Dublin S, Anderson ML, Haneuse SJ, Heckbert SR, Crane PK, Breitner JC, et al. Atrial fibrillation and risk of dementia: a prospective cohort study. J Am Geriatr Soc 2011;59(8):1369-1375ArticlePubMedPMC
- 10. Singh-Manoux A, Fayosse A, Sabia S, Canonico M, Bobak M, Elbaz A, et al. Atrial fibrillation as a risk factor for cognitive decline and dementia. Eur Heart J 2017;38(34):2612-2618ArticlePubMedPMCPDF
- 11. Miyasaka Y, Barnes ME, Petersen RC, Cha SS, Bailey KR, Gersh BJ, et al. Risk of dementia in stroke-free patients diagnosed with atrial fibrillation: data from a community-based cohort. Eur Heart J 2007;28(16):1962-1967ArticlePubMedPDF
- 12. Wolf PA, Abbott RD, Kannel WB. Atrial fibrillation as an independent risk factor for stroke: the Framingham Study. Stroke 1991;22(8):983-988ArticlePubMed
- 13. Aldrugh S, Sardana M, Henninger N, Saczynski JS, McManus DD. Atrial fibrillation, cognition and dementia: a review. J Cardiovasc Electrophysiol 2017;28(8):958-965ArticlePubMedPMC
- 14. Stirling J, Muramatsu K, Shirai T. Cerebral embolism as a cause of stroke and transient ischemic attack. Echocardiography 1996;13(5):513-518ArticlePubMed
- 15. Alosco ML, Spitznagel MB, Sweet LH, Josephson R, Hughes J, Gunstad J. Atrial fibrillation exacerbates cognitive dysfunction and cerebral perfusion in heart failure. Pacing Clin Electrophysiol 2015;38(2):178-186ArticlePubMed
- 16. Conway DS, Lip GY. Inflammation, arrhythmia burden and the thrombotic consequences of atrial fibrillation. Eur Heart J 2004;25(19):1761ArticlePDF
- 17. Choudhury A, Lip GY. Atrial fibrillation and the hypercoagulable state: from basic science to clinical practice. Pathophysiol Haemost Thromb 2003;33(5-6):(282):289Article
- 18. Stefansdottir H, Arnar DO, Aspelund T, Sigurdsson S, Jonsdottir MK, Hjaltason H, et al. Atrial fibrillation is associated with reduced brain volume and cognitive function independent of cerebral infarcts. Stroke 2013;44(4):1020-1025ArticlePubMedPMC
- 19. Bunch TJ, Crandall BG, Weiss JP, May HT, Bair TL, Osborn JS, et al. Patients treated with catheter ablation for atrial fibrillation have long-term rates of death, stroke, and dementia similar to patients without atrial fibrillation. J Cardiovasc Electrophysiol 2011;22(8):839-845ArticlePubMed
- 20. Knecht S, Oelschläger C, Duning T, Lohmann H, Albers J, Stehling C, et al. Atrial fibrillation in stroke-free patients is associated with memory impairment and hippocampal atrophy. Eur Heart J 2008;29(17):2125-2132ArticlePubMedPDF
- 21. Kim YJ, Han JW, So YS, Seo JY, Kim KY Kim KW. Prevalence and trends of dementia in Korea: a systematic review and meta-analysis. J Korean Med Sci 2014;29(7):903-912ArticlePubMedPMC
- 22. Shen AY, Yao JF, Brar SS, Jorgensen MB, Chen W. Racial/ethnic differences in the risk of intracranial hemorrhage among patients with atrial fibrillation. J Am Coll Cardiol 2007;50(4):309-315ArticlePubMed
- 23. Ugowe FE, Jackson LR II, Thomas KL. Racial and ethnic differences in the prevalence, management, and outcomes in patients with atrial fibrillation: a systematic review. Heart Rhythm 2018;15(9):1337-1345ArticlePubMed
- 24. Plassman BL, Langa KM, Fisher GG, Heeringa SG, Weir DR, Ofstedal MB, et al. Prevalence of dementia in the United States: the aging, demographics, and memory study. Neuroepidemiology 2007;29(1-2):125-132ArticlePubMedPMC
- 25. Rodriguez CJ, Soliman EZ, Alonso A, Swett K, Okin PM, Goff DC Jr, et al. Atrial fibrillation incidence and risk factors in relation to race-ethnicity and the population attributable fraction of atrial fibrillation risk factors: the Multi-Ethnic Study of Atherosclerosis. Ann Epidemiol 2015;25(2):71-76ArticlePubMed
- 26. Heeringa J, Kors JA, Hofman A, van Rooij FJ, Witteman JC. Cigarette smoking and risk of atrial fibrillation: the Rotterdam Study. Am Heart J 2008;156(6):1163-1169ArticlePubMed
- 27. Frost L, Vestergaard P. Alcohol and risk of atrial fibrillation or flutter: a cohort study. Arch Intern Med 2004;164(18):1993-1998ArticlePubMed
- 28. Peters R, Poulter R, Warner J, Beckett N, Burch L, Bulpitt C. Smoking, dementia and cognitive decline in the elderly, a systematic review. BMC Geriatr 2008;8: 36ArticlePubMedPMCPDF
- 29. Reiman EM, Chen K, Alexander GE, Caselli RJ, Bandy D, Osborne D, et al. Functional brain abnormalities in young adults at genetic risk for late-onset Alzheimer’s dementia. Proc Natl Acad Sci U S A 2004;101(1):284-289ArticlePubMed
- 30. Darbar D, Herron KJ, Ballew JD, Jahangir A, Gersh BJ, Shen WK, et al. Familial atrial fibrillation is a genetically heterogeneous disorder. J Am Coll Cardiol 2003;41(12):2185-2192ArticlePubMed
- 31. Lai LP, Su MJ, Yeh HM, Lin JL, Chiang FT, Hwang JJ, et al. Association of the human minK gene 38G allele with atrial fibrillation: evidence of possible genetic control on the pathogenesis of atrial fibrillation. Am Heart J 2002;144(3):485-490ArticlePubMed
- 32. Benjamin EJ, Levy D, Vaziri SM, D’Agostino RB, Belanger AJ, Wolf PA. Independent risk factors for atrial fibrillation in a population-based cohort. The Framingham Heart Study. JAMA 1994;271(11):840-844ArticlePubMed
- 33. Son MK, Lim NK, Cho MC, Park HY. Incidence and risk factors for atrial fibrillation in Korea: the National Health Insurance Service Database (2002–2010). Korean Circ J 2016;46(4):515-521ArticlePubMedPMC
- 34. Luchsinger JA, Reitz C, Honig LS, Tang MX, Shea S, Mayeux R. Aggregation of vascular risk factors and risk of incident Alzheimer disease. Neurology 2005;65(4):545-551ArticlePubMedPMC
- 35. Kalantarian S, Stern TA, Mansour M, Ruskin JN. Cognitive impairment associated with atrial fibrillation: a meta-analysis. Ann Intern Med 2013;158(5 Pt 1):338-346ArticlePubMedPMC
- 36. Santangeli P, Di Biase L, Bai R, Mohanty S, Pump A, Cereceda Brantes M, et al. Atrial fibrillation and the risk of incident dementia: a meta-analysis. Heart Rhythm 2012;9(11):1761-1768ArticlePubMed
- 37. Nishtala A, Piers RJ, Himali JJ, Beiser AS, Davis-Plourde KL, Saczynski JS, et al. Atrial fibrillation and cognitive decline in the Framingham Heart Study. Heart Rhythm 2018;15(2):166-172ArticlePubMed
- 38. Kim D, Yang PS, Yu HT, Kim TH, Jang E, Sung JH, et al. Risk of dementia in stroke-free patients diagnosed with atrial fibrillation: data from a population-based cohort. Eur Heart J 2019;40(28):2313-2323ArticlePubMedPDF
- 39. Friberg L, Rosenqvist M. Less dementia with oral anticoagulation in atrial fibrillation. Eur Heart J 2018;39(6):453-460ArticlePubMedPDF
- 40. Lee SR, Choi EK, Han KD, Cha MJ, Oh S. Trends in the incidence and prevalence of atrial fibrillation and estimated thromboembolic risk using the CHA2DS2-VASc score in the entire Korean population. Int J Cardiol 2017;236: 226-231ArticlePubMed
REFERENCES
Figure & Data
References
Citations

- Effects of risk factors on the development and mortality of early- and late-onset dementia: an 11-year longitudinal nationwide population-based cohort study in South Korea
Min Young Chun, Wonjeong Chae, Sang Won Seo, Hyemin Jang, Jihwan Yun, Duk L. Na, Dongwoo Kang, Jungkuk Lee, Dustin B. Hammers, Liana G. Apostolova, Sung-In Jang, Hee Jin Kim
Alzheimer's Research & Therapy.2024;[Epub] CrossRef - The effect of oral anticoagulants on the incidence of dementia in patients with atrial fibrillation: A systematic review and meta-analysis
Fakhar Latif, Muhammad Moiz Nasir, Komail K. Meer, Syed Husain Farhan, Huzaifa Ahmad Cheema, Adam Bilal Khan, Mohammad Umer, Wajeeh Ur Rehman, Adeel Ahmad, Muhammad Aslam Khan, Talal Almas, Sebastian Mactaggart, Abdulqadir J. Nashwan, Raheel Ahmed, Sourbh
International Journal of Cardiology Cardiovascular Risk and Prevention.2024; 21: 200282. CrossRef - Progressive Memory Decline in a Patient With Atrial Septal Defect: Case Report and Literature Review
Yaw Amo Wiafe, Gordon Manu Amponsah, George Asafu Adjaye Frimpong, Isaac Kofi Owusu
Clinical Medicine Insights: Case Reports.2023; 16: 117954762311767. CrossRef - FIBRILAÇÃO ATRIAL E DEMÊNCIA VASCULAR: UMA REVISÃO INTEGRATIVA DA LITERATURA
Caroline Melo de Sousa, Milena Nunes Alves Sousa, Fabrício Kleber de Lucena Carvalho
Revista Contemporânea.2022; 2(3): 739. CrossRef - A systematic review and meta‐analysis to determine the effect of oral anticoagulants on incidence of dementia in patients with atrial fibrillation
Mingjie Lin, Wenqiang Han, Jingquan Zhong, Lin Wu
International Journal of Clinical Practice.2021;[Epub] CrossRef - Risk of dementia in patients with atrial fibrillation: Short versus long follow‐up. A systematic review and meta‐analysis
Marco Zuin, Loris Roncon, Angelina Passaro, Cristina Bosi, Carlo Cervellati, Giovanni Zuliani
International Journal of Geriatric Psychiatry.2021; 36(10): 1488. CrossRef - Clinical Utility of the Pathogenesis-Related Proteins in Alzheimer’s Disease
Bin Zhou, Masanori Fukushima
International Journal of Molecular Sciences.2020; 21(22): 8661. CrossRef
- Figure
- Related articles
-
- Association Between Objective Social Isolation and Unmet Medical Needs: A Nationwide Cross-sectional Study in Korea
- Association Between Tobacco Smoking and Dental Caries in the Indonesian Population: Results of a National Study in 2018
- The Association Between Metabolic Syndrome and Colorectal Cancer Risk by Obesity Status in Korean Women: A Nationwide Cohort Study
 KSPM
KSPM

 PubReader
PubReader ePub Link
ePub Link Cite
Cite