Articles
- Page Path
- HOME > J Prev Med Public Health > Volume 54(1); 2021 > Article
-
Original Article
Associations Between Thyroid Hormone Levels and Urinary Concentrations of Bisphenol A, F, and S in 6-Year-old Children in Korea -
Yoonyoung Jang1,2
 , Yoon-Jung Choi1,2
, Yoon-Jung Choi1,2 , Youn-Hee Lim3,4
, Youn-Hee Lim3,4 , Kyung-Shin Lee1,2
, Kyung-Shin Lee1,2 , Bung-Nyun Kim5
, Bung-Nyun Kim5 , Choong Ho Shin6
, Choong Ho Shin6 , Young Ah Lee6
, Young Ah Lee6 , Johanna Inhyang Kim7
, Johanna Inhyang Kim7 , Yun-Chul Hong1,2,3
, Yun-Chul Hong1,2,3
-
Journal of Preventive Medicine and Public Health 2021;54(1):37-45.
DOI: https://doi.org/10.3961/jpmph.20.310
Published online: November 23, 2020
1Department of Preventive Medicine, Seoul National University College of Medicine, Seoul, Korea
2Environmental Health Center, Seoul National University College of Medicine, Seoul, Korea
3Institute of Environmental Medicine, Seoul National University Medical Research Center, Seoul, Korea
4Section of Environmental Health, Department of Public Health, University of Copenhagen, Copenhagen, Denmark
5Division of Child and Adolescent Psychiatry, Department of Psychiatry, Seoul National University College of Medicine, Seoul, Korea
6Department of Pediatrics, Seoul National University College of Medicine, Seoul, Korea
7Department of Psychiatry, Hanyang University Medical Center, Seoul, Korea
- Corresponding author: Yun-Chul Hong, Institute of Environmental Medicine, Seoul National University Medical Research Center, 103 Daehakro, Jongno-gu, Seoul 03080, Korea, E-mail: ychong1@snu.ac.kr
Copyright © 2021 The Korean Society for Preventive Medicine
This is an Open Access article distributed under the terms of the Creative Commons Attribution Non-Commercial License (https://creativecommons.org/licenses/by-nc/4.0/) which permits unrestricted non-commercial use, distribution, and reproduction in any medium, provided the original work is properly cited.
ABSTRACT
-
Objectives
- Bisphenol A (BPA) is used in the electrical, mechanical, medical, and food industries. Previous studies have suggested that BPA is an endocrine disruptor. Regulation of BPA has led to increased use of bisphenol F (BPF) and bisphenol S (BPS). However, few studies have investigated the associations of BPF and BPS with thyroid dysfunction in children. Our study investigated the associations of prenatal BPA and early childhood BPA, BPF, and BPS exposure with thyroid function in 6-year-old children.
-
Methods
- Prenatal BPA concentrations were measured during the second trimester of pregnancy in an established prospective birth cohort. We measured urinary BPA, BPF, and BPS concentrations and thyroid hormone levels (thyroid-stimulating hormone, total T3, and free T4) in 6-year-old children (n=574). We examined the associations between urinary bisphenol concentrations and percentage change of thyroid hormone concentrations using multivariate linear regression. We also compared thyroid hormone levels by dividing the cohort according to BPA, BPF, and BPS concentrations.
-
Results
- The associations between prenatal BPA and total T3 levels were statistically significant in all models, except for girls when using a crude model. The associations between urinary BPA and BPS concentrations and levels of all thyroid hormones were not statistically significant. However, we observed that lower free T4 levels (−1.94%; 95% confidence interval, −3.82 to −0.03) were associated with higher urinary BPF concentrations in girls only.
-
Conclusions
- Our findings identified significant associations between prenatal BPA exposure and total T3 levels in all children and between BPF exposure and free T4 levels in girls only.
- Bisphenols (BPs) are used to manufacture widely used plastics such as epoxy resins and polycarbonate polymers. BPs are detected ubiquitously in surface water, sediments, and dirt, and are prevalently detected in human blood, urine, placental tissue, and breast milk [1]. Over the past few decades, it has been reported that exposure to endocrine-disrupting chemicals, such as bisphenol A (BPA), during vulnerable periods including childhood may be related to a wide variety of health effects, encompassing behavioral changes [2–6], social impairment [7], early puberty [8], cardiovascular effects such as elevated blood pressure [9–12], and respiratory problems [13].
- As a result of consumer concerns and accumulating scientific evidence, plastic manufacturers have started to eliminate BPA from their products. Consequently, there has been a steady switch to BP analogues [14] as alternatives, leading to the replacement of BPA with bisphenol F (BPF) or bisphenol S (BPS). BPF is used in the production of tanks and pipes, industrial floors, road and bridge deck toppings, structural adhesives, grouts, coatings, and electrical varnishes, as well as in various consumer products such as lacquers, varnishes, liners, adhesives, water pipes, dental sealants, and food packaging [15]. BPS is used for a variety of industrial applications, such as cleaning products and electroplating solvents [16]. BPS is also used as an alternative in thermal papers labeled as “BPA-free” [17]. A recent review article suggested that BPS is as harmful as BPA with respect to obesogenic effects, preadipocyte stimulation, metabolic disorders such as gestational diabetes, and reproductive system disorders including breast cancer [18]. The effects of BPF and BPS in inducing androgenic, antiandrogenic, and estrogenic effects; cytotoxicity; genotoxicity; and mutagenicity have been established through several in vivo and in vitro studies [14]. However, few studies have investigated the effect of BPF or BPS exposure on humans, especially children.
- Thyroid hormone maintains homeostasis in our body, helps to synthesize proteins, maintains cardiovascular functions, promotes the growth of the central nervous and skeletal systems, and regulates hematopoietic functions. Several in vitro and animal studies have provided evidence for an association between BPA exposure and the disruption of thyroid hormone [19,20]. BPA has been found to interfere with the expression of thyroid function-related genes in zebrafish embryos (in vivo) and FRTL-5 cells, an immortalized thyroid follicular cell line derived from normal rat thyroid (in vitro), by dysregulating the expression of thyroid transcription factors such as Pax8, Nkx2-1, and Foxe1 [21–24]. In addition, BPA inhibits the sodium/iodide symporter, which affects the signaling and actions of thyroid hormone [23].
- Many studies have reported several effects of prenatal BPA exposure on fetal growth [25], diastolic blood pressure [9], dysregulation of hypothalamic-pituitary-adrenal axis function in newborns, and alteration of hormone levels including cortisol [26]. Significant effects of BPs on the behavior [3] and emotions [2] of school-aged children have also been reported in a sex-specific manner. However, the sex-specific effects of BPF or BPS on thyroid function in children remain unknown.
- In this study, we investigated the associations of prenatal BPA and early childhood BPA, BPF, and BPS exposure with thyroid function in 6-year-old children. Moreover, we investigated the sex-specific relationship of BPs with thyroid hormone levels.
INTRODUCTION
- Study Population
- This study was based on a prospective cohort study, the Environment and Development of Children Study, designed to investigate environmental effects on the growth and development of children. The participants were mothers and children who previously participated in another prospective cohort study of birth outcomes, the Congenital Anomaly Study (CAS). Details of the CAS have been previously described [7,9,27]. In brief, we contacted 2085 mothers selected randomly from the 10 752 CAS participants, and 726 mother-child pairs were registered in the present study (response rate, 31%). We conducted follow-up assessments when the children were approximately 6 years of age, between March 2015 and December 2017. The children underwent health examinations at the Seoul National University Hospital in Jongno-gu, Seoul, Korea.
- At the follow-up examination, urine and blood sample collections, as well as physical examinations, were conducted after the participants fasted for more than 8 hours. In total, 574 children were included in the final analyses.
- Exposure
- Our study had 2 time points: prenatal BPA exposure and postnatal BPA, BPF, and BPS exposure. Prenatal urinary BPA values were obtained from the mothers and postnatal urinary BPA, BPF, and BPS values were obtained from the 6-year-old children during the follow-up assessments. Maternal spot urine samples were collected during the second trimester of pregnancy (mean, 20 weeks). Children’s urine samples were collected after a minimum 8-hour fast and the collected samples were stored at −20°C. We measured the total concentrations (free and conjugated species) of urinary BPA in mothers and urinary BPA, BPF, and BPS in children. BPA, BPF and BPS become water-soluble in the form of glucuronide and sulfate conjugates. These were hydrolyzed with β-glucuronidase, extracted with methyl tertiary butyl ether, concentrated, dried, and dissolved in 300 μL of 60% acetonitrile. BPA was quantified using high-performance liquid chromatography-tandem mass spectrometry (Agilent 6410 Triple Quad LCMS; Agilent Technologies, Santa Clara, CA, USA), as described previously [9,28,29]. The concentrations of BPF and BPS were quantified using an Agilent 6490 Triple Quad LCMS (Agilent Technologies). The lower limit of detection (LOD) for BPA ranged from 0.031 μg/L to 0.212 μg/L depending on the batch; therefore, we used the highest value of 0.212 μg/L, and that of BPF and BPS was 0.074 μg/L and 0.020 μg/L, respectively. The method detection limit was used to measure LOD. At first, in order to perform a generalized linear regression model (GLM), the value of the LOD divided by the square root of 2 was substituted for BPF and BPS levels below the LOD [30]. Later, we also divided BPF and BPS levels into 3 groups based on categorical values and compared the mean of each group because of the low detection rate of BPF and BPS (23.6 and 42.2%, respectively).
- We used creatinine-adjusted prenatal BPA concentrations in the analysis. However, when we analyzed BPA, BPF, and BPS concentrations in the 6-year-old children, we did not adjust for urinary creatinine concentrations because they are unstable due to rapid changes in muscle mass during growth and development [31]. The urinary BPA, BPF, and BPS concentrations of children at the time of follow-up (approximately at age 6) were measured using the same method used to measure prenatal urinary BPA. All BPA, BPF, and BPS exposures were natural log-transformed because of the skewedness of the data.
- Children’s blood samples were also collected after a minimum 8-hour fast. The thyroid function test (thyroid-stimulating hormone [TSH], total T3 [T3], and free T4 [fT4]) was performed using chemiluminescent microparticle immunoassay analysis on an Architect i2000 analyzer (Abbott Korea, Seoul, Korea). The normal reference ranges of TSH, T3, and fT4 were 0.38–4.94 μIU/mL, 58–159 ng/dL, and 0.70–1.48 ng/dL, respectively. Thyroid hormone concentrations were natural log-transformed for analysis.
- Covariates
- The models included potential covariates that are known or suspected risk factors for BP concentrations and health effects in children [2,4,7]. At the time of recruitment, we gathered prenatal information on maternal age (years), gestational age (weeks), maternal educational level (≤ or >high school), parity (first vs. second or later child), and household income (monthly). Children’s characteristics such as age (months), sex, body mass index (BMI, kg/m2), birth weight (kg), and exposure to secondhand tobacco smoking (yes or no) were obtained at the follow-up visit.
- Statistical Analysis
- Urinary BP concentrations were transformed using the natural logarithm to account for the skewedness of the collected data. We assessed the association of log-transformed urinary BP concentrations with log-transformed serum thyroid hormone concentrations and the relationship between log-transformed prenatal BPA and log-transformed thyroid hormone levels in 6-year-old children using a GLM.
- Potential covariates were included in the statistical models. We established several statistical models to find the model that best described the relationship between BPs and thyroid hormone levels. Model 1 adjusted for age, sex, and BMI, while model 2 adjusted for maternal age, education level, parity, and gestational weeks in addition to the covariates used in model 1. Model 3 further adjusted for monthly household income, exposure to secondhand smoking, and children’s urinary creatinine levels in addition to the covariates used in model 2. Finally, model 4 adjusted for the same covariates used in model 3 except for parity and gestational weeks. Model 4 was the main model used in this study. To investigate the relationships with prenatal BPA, we applied the prenatal BPA values in each model, and to investigate the relationships with BPA, BPF, and BPS exposure in 6-year-old children, we applied the urinary BPA, BPF, and BPS values of the 6-year-old children in each model. In order to examine potential sex-based difference, we stratified our samples by the sex of the child. We created an additional model that included interaction terms (urinary BP concentrations*child sex, considered significant at p<0.10) to justify the statistical basis for sex stratification.
- To compensate for the low detection rates of BPF and BPS, we investigated differences in the mean thyroid hormone concentrations according to 3 groups of BPF and BPS levels using analysis of variance (ANOVA); children included in the lowest group were those with BPF levels ≤75th percentile and BPS levels ≤50th percentile, and children in the middle group were those with BPF levels >75th percentile and ≤90th percentile and BPS levels >50th percentile and ≤90th percentile. Children in the highest group were those with BPF levels >90th percentile and BPS levels >90th percentile.
- The statistical analyses were conducted using SAS version 9.4 (SAS Institute Inc., Cary, NC, USA) and R version 3.2.1 ( https://cran.r-project.org/bin/windows/base/old/3.2.1/). Two-tailed p-values of less than 0.05 were considered to indicate statistical significance.
- Ethics Statement
- We obtained informed consent from all participants, and the study protocol was approved by the Institutional Review Board of the Seoul National University College of Medicine (No. 1201-010-392).
METHODS
- The characteristics of the study subjects with respect to age, sex, BMI, secondhand smoking, maternal age, maternal education level, monthly household income, gestational week, parity and thyroid function test results are presented in Table 1. There were more boys than girls (52.3 and 47.7% respectively), and the mean age of the children was 71.1±1.5 months. The mean BMI of the children was 15.8±1.8 kg/m2. Most children in the study were not exposed to secondhand smoking (76.3%). The mean maternal age was 31.4±3.6 years. The children’s mean gestational age was 38.6±1.6 weeks and 59.6% of children were the first children in the family. The mean levels of TSH, T3, and fT4 among the children were 2.55±1.36 μIU/mL, 148.00±18.47 ng/dL, and 1.15±0.11 ng/dL, respectively. TSH and T3 levels were slightly higher in girls. However, fT4 levels were slightly lower in girls.
- Our additional model (p<0.10) to investigate the interaction between child sex and urinary BP concentrations showed no significant results.
- The highest urinary BP concentration in children was found for BPA (2.73±7.15 μg/L), followed by BPS (0.21±1.44 μg/L) and BPF (0.11±0.22 μg/L) (Table 2). The mean urinary levels of BPA and BPF were higher in boys, whereas the mean level of BPS was higher in girls. However, the median level of BPF (0.05 μg/L) and BPS (0.01 μg/L) was the same in both sexes. The mean value of creatinine-adjusted prenatal BPA was 2.15±2.85 μg/g Cr, and the value for boys was higher.
- The relationship between prenatal BPA and T3 (Supplemental Materials 1–5) was significant only when stratified by sex, but there was no significant result in the crude model for girls.
- Table 3 shows the absence of significant associations between BPA and BPS and thyroid hormone levels. However, higher urinary BPF concentrations in girls were significantly associated with lower fT4 levels (model 4; β=−0.02; standard error =0.01; p<0.05). Figure 1 shows the association of fT4 concentrations with each urinary BP analogue in 6-year-old children. Only BPF showed a significant negative association with fT4 in girls. The detailed values presented in Figure 1 are shown in Supplemental Material 6.
- We did not find any significant results (p<0.10) in ANOVA comparing the mean concentrations of thyroid hormones among the lowest, middle, and highest BPS groups. However, we found that the comparison of concentrations of TSH and fT4 according to the BPF level (low, middle, and high) yielded statistically significant results at a significance level of 10% (Supplemental Materials 7 and 8).
RESULTS
- We investigated the associations between urinary BP concentrations at 2 time points (prenatal and at 6 years old) and thyroid hormone levels in 6-year-old children. The association between prenatal BPA and T3 levels in 6-year-old children was statistically significant in a sex-specific manner in all models except for the crude model. The association between postnatal BPF and fT4 was statistically significant among girls, but not boys. Therefore, we suggest that prenatal BPA exposure and postnatal BPF exposure in 6-year-old children are associated with thyroid function in a sex-specific manner.
- The sex-specific effects of BPA exposure on hormonal homeostasis, sexual development, and growth may result in its estrogenic and androgenic effects, which have been documented in previous animal studies [32–34]. Similarly, the sexually dimorphic phenotypic effects of BP exposure on hormonal homeostasis, obesity, behavior, and anxiety may be explained by epigenetic mechanisms [35].
- Moreover, considering the effects of thyroid hormone on aspects of health, such as the cardiovascular system [36], our results might be helpful, at least to some extent, in determining the possible mechanism of action of BPs for previously discussed health issues such as blood pressure, which is related to thyroid hormonal function.
- Rochester and Bolden [14] studied the hormonal potency of BPF and BPS. They revealed that these substances exert estrogenic, antiestrogenic, androgenic, and antiandrogenic effects; furthermore, they found that the potency of BPF and BPS in terms of aryl hydrocarbon activity and inhibitory hormonal signaling in adipocytes was of the same order of magnitude as the potency of BPA. A noteworthy finding of their study was that BPF may have a strong potential for hormonal activity, similar to or greater than that of BPA. For example, the average estrogenic potency was 1.07±1.20 (mean±standard deviation) compared with BPA, while that of BPS compared with BPA was 0.32±0.28.
- Thyroid hormone is important for growth, development, and vital function in children, and is produced by the thyroid gland in the form of T4 or T3. TSH, which is produced in the anterior lobe of the pituitary gland, promotes the entire process from iodine uptake to the synthesis and secretion of thyroid hormone. TSH promotes the secretion of T3 and T4, which inhibit the excess secretion of TSH through a negative feedback mechanism.
- A distinct characteristic of thyroid hormone is that it affects almost all organs in the human body. Since it is involved in long-term biological processes, such as growth, maturation, and adaptation, the early detection of abnormal thyroid function is difficult in terms of clinical manifestations. Thyroid hormones are essential for the normal growth and maturation of skeletal muscle. Hyperthyroidism causes goiter, exophthalmos, an increase in basal metabolism, and emotional instability. In contrast, low levels of thyroid function during growth suppress the growth of skeletal tissue and the development processes of the body.
- In addition, previous studies have suggested that the consistent tendency of prenatal BPA exposure to decrease T3 in boys and increase T3 in girls reflects its disruption of thyroid function [6,37,38]. However, Sanlidag et al. [26] showed that there was no significant relationship between cord blood BPA and newborn thyroid hormone levels. Further research, including large-population studies and meta-analyses, is needed to determine whether an effect really exists.
- Since evidence for the health effects of BPS and BPF remains scarce, their effects can only be inferred from existing in vitro or in vivo studies. While it is difficult to draw a conclusion about the effects of BPs on thyroid hormone from a previous systematic literature review [14], Higashihara et al. [39] showed that BPF affected thyroid gland weight and thyroid hormone concentrations. In our study, the finding of a relationship between BPF exposure and lower fT4 levels in girls suggests that BPF exposure in children may place them at risk for diminished thyroid function, which is critical in growth and development.
- Our study has some strengths worth noting. First, our study was conducted as a prospective cohort study, reducing the potential effect of recall bias. Second, to our best knowledge, this is the first study of the association between prenatal and postnatal BP exposure and thyroid hormones in preschool-aged children. Our findings show that exposure to BPs in any period may affect children’s thyroid function.
- Our study also has some limitations. First, in this study, we could not analyze calcitonin, which modulates calcium (Ca2+) metabolism alongside parathyroid hormone, although Ca2+ is also involved in the blood circulation, as well as the musculoskeletal and nervous systems [40]. Second, even though iodine uptake is an important component of thyroid hormone production, we were not able to measure blood iodine concentrations in the children. Finally, the fact that the detection rate of BPF and BPS was low limited our ability to establish links between exposure to BP analogues and thyroid hormone concentrations. Therefore, we grouped BPF and BPS values into 3 groups (lowest, middle, and highest) and explored the relationship of BPF and BPS concentrations as categorical variables with thyroid hormone levels.
- To our knowledge, this is the first study to suggest an association between BPF and fT4 in 6-year-old girls. Exposure to BPF may cause estrogenic and thyroidogenic effects and disrupt thyroid hormone in girls. Since levels of BP analogues in the human body have increased over time, the use of BP analogues in consumer goods, especially those targeted at children, should be reconsidered.
DISCUSSION
SUPPLEMENTAL MATERIALS
-
CONFLICT OF INTEREST
The authors have no conflicts of interest associated with the material presented in this paper.
-
FUNDING
This research was supported by a grant from the Center for Environmental Health through the Ministry of Environment and from the Ministry of Food and Drug Safety in 2018 (18162-MFDS121).
-
AUTHOR CONTRIBUTIONS
Conceptualization: YJ, YCH. Data curation: YHL, BNK, CHS, YAL, JIK, YCH. Formal analysis: YJ, YJC, KSL, YHL, YCH. Funding acquisition: YCH. Methodology: YJ, YHL, YCH. Project administration: YCH. Visualization: YCH. Writing – original draft: YJ. Writing – review & editing: YJ, YJC, KSL, YCH, YHL, BNK, CHS, YAL, JIK.
Notes
ACKNOWLEDGEMENTS
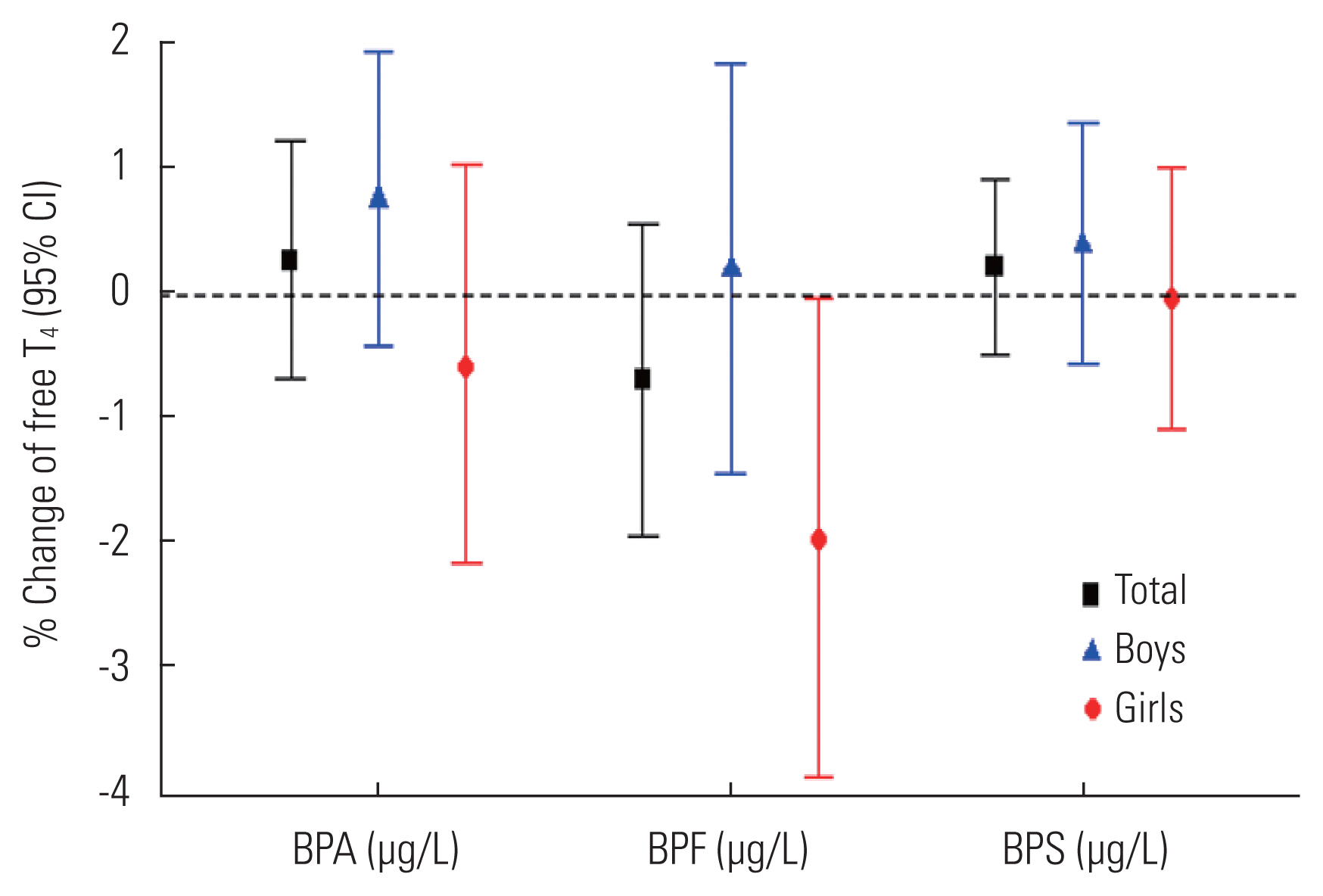
Model 4 was adjusted for age (months), sex, body mass index, mother’s age, mother’s education, monthly household income, secondhand smoke, urinary creatinine level.
TSH, thyroid-stimulating hormone; BP, bisphenol; T3, total T3; fT4, free T4; SE, standard error; BPA, bisphenol A; BPF, bisphenol F; BPS, bisphenol S; Cr, creatinine.
- 1. Lee J, Choi K, Park J, Moon HB, Choi G, Lee JJ, et al. Bisphenol A distribution in serum, urine, placenta, breast milk, and umbilical cord serum in a birth panel of mother-neonate pairs. Sci Total Environ 2018;626: 1494-1501ArticlePubMed
- 2. Harley KG, Gunier RB, Kogut K, Johnson C, Bradman A, Calafat AM, et al. Prenatal and early childhood bisphenol A concentrations and behavior in school-aged children. Environ Res 2013;126: 43-50ArticlePubMedPMC
- 3. Roen EL, Wang Y, Calafat AM, Wang S, Margolis A, Herbstman J, et al. Bisphenol A exposure and behavioral problems among inner city children at 7–9 years of age. Environ Res 2015;142: 739-745ArticlePubMedPMC
- 4. Braun JM, Muckle G, Arbuckle T, Bouchard MF, Fraser WD, Ouellet E, et al. Associations of prenatal urinary bisphenol A concentrations with child behaviors and cognitive abilities. Environ Health Perspect 2017;125(6):067008ArticlePubMedPMC
- 5. Ghassabian A, Bell EM, Ma WL, Sundaram R, Kannan K, Buck Louis GM, et al. Concentrations of perfluoroalkyl substances and bisphenol A in newborn dried blood spots and the association with child behavior. Environ Pollut 2018;243(Pt B):1629-1636ArticlePubMedPMC
- 6. Li F, Yang F, Li DK, Tian Y, Miao M, Zhang Y, et al. Prenatal bisphenol A exposure, fetal thyroid hormones and neurobehavioral development in children at 2 and 4 years: a prospective cohort study. Sci Total Environ 2020;722: 137887ArticlePubMed
- 7. Lim YH, Bae S, Kim BN, Shin CH, Lee YA, Kim JI, et al. Prenatal and postnatal bisphenol A exposure and social impairment in 4-year-old children. Environ Health 2017;16(1):79ArticlePubMedPMC
- 8. Berger K, Eskenazi B, Kogut K, Parra K, Lustig RH, Greenspan LC, et al. Association of prenatal urinary concentrations of phthalates and bisphenol A and pubertal timing in boys and girls. Environ Health Perspect 2018;126(9):97004ArticlePubMed
- 9. Bae S, Lim YH, Lee YA, Shin CH, Oh SY, Hong YC. Maternal urinary bisphenol A concentration during midterm pregnancy and children’s blood pressure at age 4. Hypertension 2017;69(2):367-374ArticlePubMed
- 10. Sanders AP, Saland JM, Wright RO, Satlin L. Perinatal and childhood exposure to environmental chemicals and blood pressure in children: a review of literature 2007–2017. Pediatr Res 2018;84(2):165-180ArticlePubMedPMC
- 11. Amin MM, Ebrahim K, Hashemi M, Shoshtari-Yeganeh B, Rafiei N, Mansourian M, et al. Association of exposure to bisphenol A with obesity and cardiometabolic risk factors in children and adolescents. Int J Environ Health Res 2019;29(1):94-106ArticlePubMed
- 12. Warembourg C, Maitre L, Tamayo-Uria I, Fossati S, Roumeliotaki T, Aasvang GM, et al. Early-life environmental exposures and blood pressure in children. J Am Coll Cardiol 2019;74(10):1317-1328ArticlePubMedPMC
- 13. Buckley JP, Quirós-Alcalá L, Teitelbaum SL, Calafat AM, Wolff MS, Engel SM. Associations of prenatal environmental phenol and phthalate biomarkers with respiratory and allergic diseases among children aged 6 and 7 years. Environ Int 2018;115: 79-88ArticlePubMedPMC
- 14. Rochester JR, Bolden AL. Bisphenol S and F: a systematic review and comparison of the hormonal activity of bisphenol a substitutes. Environ Health Perspect 2015;123(7):643-650ArticlePubMedPMC
- 15. Cabaton N, Chagnon MC, Lhuguenot JC, Cravedi JP, Zalko D. Disposition and metabolic profiling of bisphenol F in pregnant and nonpregnant rats. J Agric Food Chem 2006;54(26):10307-10314ArticlePubMed
- 16. Clark E. Sulfolane and sulfones. In: Kirk RE, Othmer DF, editors. Kirk-Othmer encyclopedia of chemical technology. New York: Wiley; 2000. p. 4-6Article
- 17. Liao C, Liu F, Kannan K. Bisphenol S, a new bisphenol analogue, in paper products and currency bills and its association with bisphenol A residues. Environ Sci Technol 2012;46(12):6515-6522ArticlePubMed
- 18. Thoene M, Dzika E, Gonkowski S, Wojtkiewicz J. Bisphenol S in food causes hormonal and obesogenic effects comparable to or worse than bisphenol A: a literature review. Nutrients 2020;12(2):532ArticlePubMedPMC
- 19. Boas M, Feldt-Rasmussen U, Main KM. Thyroid effects of endocrine disrupting chemicals. Mol Cell Endocrinol 2012;355(2):240-248ArticlePubMed
- 20. Tilley SK, Fry RC. Hormone response pathways as responders to environmental contaminants and their roles in disease. In: Fry RC, editor. Systems biology in toxicology and environmental health. Boston: Academic Press; 2015. p. 225-238Article
- 21. Wu Y, Beland FA, Fang JL. Effect of triclosan, triclocarban, 2,2′, 4,4′-tetrabromodiphenyl ether, and bisphenol A on the iodide uptake, thyroid peroxidase activity, and expression of genes involved in thyroid hormone synthesis. Toxicol In Vitro 2016;32: 310-319ArticlePubMed
- 22. Lee S, Kim C, Youn H, Choi K. Thyroid hormone disrupting potentials of bisphenol A and its analogues - in vitro comparison study employing rat pituitary (GH3) and thyroid follicular (FRTL-5) cells. Toxicol In Vitro 2017;40: 297-304ArticlePubMed
- 23. Gentilcore D, Porreca I, Rizzo F, Ganbaatar E, Carchia E, Mallardo M, et al. Bisphenol A interferes with thyroid specific gene expression. Toxicology 2013;304: 21-31ArticlePubMed
- 24. Zhang DH, Zhou EX, Yang ZL. Waterborne exposure to BPS causes thyroid endocrine disruption in zebrafish larvae. PLoS One 2017;12(5):e0176927ArticlePubMedPMC
- 25. Mustieles V, Williams PL, Fernandez MF, Mínguez-Alarcón L, Ford JB, Calafat AM, et al. Maternal and paternal preconception exposure to bisphenols and size at birth. Hum Reprod 2018;33(8):1528-1537ArticlePubMedPMC
- 26. Sanlidag B, Dalkan C, Yetkin O, Bahçeciler NN. Evaluation of dose dependent maternal exposure to bisphenol A on thyroid functions in newborns. J Clin Med 2018;7(6):119ArticlePubMedPMC
- 27. Kim W, Jang Y, Lim YH, Kim BN, Shin CH, Lee YA, et al. The effect of prenatal cadmium exposure on attention-deficit/hyperactivity disorder in 6-year-old children in Korea. J Prev Med Public Health 2020;53(1):29-36ArticlePubMed
- 28. Yang M, Kim SY, Lee SM, Chang SS, Kawamoto T, Jang JY, et al. Biological monitoring of bisphenol a in a Korean population. Arch Environ Contam Toxicol 2003;44(4):546-551ArticlePubMed
- 29. Bae S, Kim JH, Lim YH, Park HY, Hong YC. Associations of bi sphenol A exposure with heart rate variability and blood pressure. Hypertension 2012;60(3):786-793ArticlePubMed
- 30. Hornung RW, Reed LD. Estimation of average concentration in the presence of nondetectable values. Appl Occup Environ Hyg 1990;5(1):46-51Article
- 31. Mian AN, Schwartz GJ. Measurement and estimation of glomerular filtration rate in children. Adv Chronic Kidney Dis 2017;24(6):348-356ArticlePubMedPMC
- 32. Santos-Silva AP, de Moura EG, Pinheiro CR, Oliveira E, Lisboa PC. Short-term and long-term effects of bisphenol A (BPA) exposure during breastfeeding on the biochemical and endocrine profiles in rats. Horm Metab Res 2018;50(6):491-503ArticlePubMed
- 33. Ullah A, Pirzada M, Jahan S, Ullah H, Razak S, Rauf N, et al. Prenatal BPA and its analogs BPB, BPF, and BPS exposure and reproductive axis function in the male offspring of Sprague Dawley rats. Hum Exp Toxicol 2019;38(12):1344-1365ArticlePubMed
- 34. Zhou B, Yang P, Deng YL, Zeng Q, Lu WQ, Mei SR. Prenatal exposure to bisphenol a and its analogues (bisphenol F and S) and ultrasound parameters of fetal growth. Chemosphere 2020;246: 125805ArticlePubMed
- 35. McCabe C, Anderson OS, Montrose L, Neier K, Dolinoy DC. Sexually dimorphic effects of early-life exposures to endocrine disruptors: sex-specific epigenetic reprogramming as a potential mechanism. Curr Environ Health Rep 2017;4(4):426-438ArticlePubMedPMC
- 36. Jabbar A, Pingitore A, Pearce SH, Zaman A, Iervasi G, Razvi S. Thyroid hormones and cardiovascular disease. Nat Rev Cardiol 2017;14(1):39-55ArticlePubMed
- 37. Sur U, Erkekoglu P, Bulus AD, Andiran N, Kocer-Gumusel B. Oxidative stress markers, trace elements, and endocrine disrupting chemicals in children with Hashimoto’s thyroiditis. Toxicol Mech Methods 2019;29(9):633-643ArticlePubMed
- 38. Derakhshan A, Shu H, Peeters RP, Kortenkamp A, Lindh CH, Demeneix B, et al. Association of urinary bisphenols and triclosan with thyroid function during early pregnancy. Environ Int 2019;133(Pt A):105123ArticlePubMed
- 39. Higashihara N, Shiraishi K, Miyata K, Oshima Y, Minobe Y, Yamasaki K. Subacute oral toxicity study of bisphenol F based on the draft protocol for the “Enhanced OECD Test Guideline no. 407”. Arch Toxicol 2007;81(12):825-832ArticlePubMed
- 40. Kibble JD. The big picture physiology: medical course & step 1 review; 2020 [cited Jul 1]. Available from: https://accessmedicine.mhmedical.com/content.aspx?bookid=2914§ionid=245544050
REFERENCES
Figure & Data
References
Citations

- Relationship between bisphenol A and autoimmune thyroid disease in women of childbearing age
Ning Yuan, Jianbin Sun, Xin Zhao, Wei Li
Frontiers in Endocrinology.2024;[Epub] CrossRef - The effect of bisphenols on sex and thyroid hormone concentrations in cord blood among newborns
Francis Manyori Bigambo, Zhaofang Chen, Wentao Yang, Qian Huang, Xu Wang
Food and Chemical Toxicology.2024; 189: 114750. CrossRef - Maternal bisphenols exposure and thyroid function in children: a systematic review and meta-analysis
Jiani Liu, Min Tian, Haiyue Qin, Danrong Chen, Sabitina Mrisho Mzava, Xu Wang, Francis Manyori Bigambo
Frontiers in Endocrinology.2024;[Epub] CrossRef - Temporal trends in risk of bisphenol A, benzophenone-3 and triclosan exposure among U.S. children and adolescents aged 6–19 years: Findings from the National Health and Nutrition Examination Survey 2005–2016
Ruiqiang Li, Wenqiang Zhan, Jingyi Ren, Fan Zhang, Xin Huang, Yuxia Ma
Environmental Research.2023; 216: 114474. CrossRef - A case-control study of urinary concentrations of bisphenol A, bisphenol F, and bisphenol S and the risk of papillary thyroid cancer
Lei Zhang, Jiahuai Zhang, Sai Fan, Yuxin Zhong, Jingguang Li, Yunfeng Zhao, Song Ni, Jiaying Liu, Yongning Wu
Chemosphere.2023; 312: 137162. CrossRef - Transient developmental exposure to low doses of bisphenol F negatively affects neurogliogenesis and olfactory behaviour in adult mice
Pieter Vancamp, Lucile Butruille, Anni Herranen, Anita Boelen, Jean-Baptiste Fini, Barbara A. Demeneix, Sylvie Remaud
Environment International.2023; 172: 107770. CrossRef - Risk Assessment of Bisphenol A in the Korean General Population
Myungsil Hwang, Seon-Joo Park, Hae-Jeung Lee
Applied Sciences.2023; 13(6): 3587. CrossRef - The Joint Effects of Bisphenols and Iodine Exposure on Thyroid during Pregnancy
Wei Lu, Zhuo Sun, Zhengyuan Wang, Mengying Qu, Zehuan Shi, Qi Song, Liping Shen, Shupeng Mai, Yuan Wang, Xinyu Hong, Jiajie Zang
Nutrients.2023; 15(15): 3422. CrossRef - Associations of exposure to bisphenol A and its substitutes with neurodevelopmental outcomes among infants at 12 months of age: A cross-sectional study
Zhuanning Xia, Cheng Lv, Yan Zhang, Rong Shi, Qi Lu, Ying Tian, Xiaoning Lei, Yu Gao
Chemosphere.2023; 341: 139973. CrossRef - Association of urinary bisphenols with thyroid function in the general population: a cross-sectional study of an industrial park in China
Yang Hu, Shiming Lai, Ying Li, Xiaodong Wu, Mingluan Xing, Xueqing Li, Dandan Xu, Yuan Chen, Jie Xiang, Ping Cheng, Xiaofeng Wang, Zhijian Chen, Hao Ding, Peiwei Xu, Xiaoming Lou
Environmental Science and Pollution Research.2023; 30(49): 107517. CrossRef - Associations of Urinary Bisphenol a, Bisphenol F, and Bisphenol S with the Risk of Papillary Thyroid Cancer: A Case-Control Study
Lei Zhang, Jiahuai Zhang, Sai Fan, Yuxin Zhong, Jingguang Li, Yunfeng Zhao, Song Ni, Jiaying Liu, Yong-Ning Wu
SSRN Electronic Journal .2022;[Epub] CrossRef - The Impact of Bisphenol A on Thyroid Function in Neonates and Children: A Systematic Review of the Literature
Diamanto Koutaki, George Paltoglou, Aikaterini Vourdoumpa, Evangelia Charmandari
Nutrients.2021; 14(1): 168. CrossRef
 KSPM
KSPM

 PubReader
PubReader ePub Link
ePub Link Cite
Cite


